
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
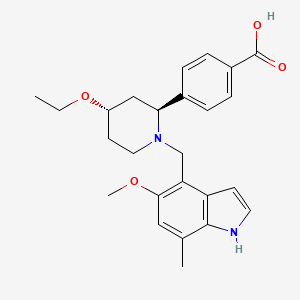

1. Lnp023
2. 1644670-37-0
3. Lnp-023
4. Iptacopan [inn]
5. Iptacopan [usan]
6. Nvp-lnp023-nx
7. Nvp-lnp023
8. 8e05t07z6w
9. 4-[(2s,4s)-4-ethoxy-1-[(5-methoxy-7-methyl-1h-indol-4-yl)methyl]piperidin-2-yl]benzoic Acid
10. 4-[(2~{s},4~{s})-4-ethoxy-1-[(5-methoxy-7-methyl-1~{h}-indol-4-yl)methyl]piperidin-2-yl]benzoic Acid
11. Benzoic Acid, 4-((2s,4s)-4-ethoxy-1-((5-methoxy-7-methyl-1h-indol-4-yl)methyl)-2-piperidinyl)-
12. Benzoic Acid, 4-[(2s,4s)-4-ethoxy-1-[(5-methoxy-7-methyl-1h-indol-4-yl)methyl]-2-piperidinyl]-
13. Iptacopan [who-dd]
14. Unii-8e05t07z6w
15. Chembl4594448
16. Schembl16400416
17. Gtpl10710
18. Us9682968, Example-26a
19. Bdbm160475
20. Ex-a5728
21. Who 11259
22. Zinc223246892
23. At30389
24. Compound 41 [pmid: 32073845]
25. Hy-127105
26. Cs-0093107
27. A935227
28. 4-((2s,4s)-(4-ethoxy-1-((5-methoxy-7-methyl-1h-indol-4-yl)methyl)piperidin-2-yl))benzoic Acid
29. 4-((2s,4s)-4-ethoxy-1-((5-methoxy-7-methyl-1h-indol-4-yl)methyl)piperidin-2-yl)benzoicacid
30. Jgq
| Molecular Weight | 422.5 g/mol |
|---|---|
| Molecular Formula | C25H30N2O4 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 422.22055744 g/mol |
| Monoisotopic Mass | 422.22055744 g/mol |
| Topological Polar Surface Area | 74.8 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 594 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
NDC Package Code : 44139-0100
Start Marketing Date : 2023-12-05
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway. It is approved for the treatment of patients with IgA nephropathy (IgAN).
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Nephrology Brand Name: Fabhalta
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Novartis Receives FDA Approval for Fabhalta, A Complement Inhibitor for IgAN
Details : Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway. It is approved for the treatment of patients with IgA nephropathy (IgAN).
Brand Name : Fabhalta
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway. It is being evaluated for the treatment of patients with IgA nephropathy (IgAN).
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Nephrology Brand Name: Fabhalta
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Novartis Data Show Significant Proteinuria Reduction of 38.3% for IgA Nephropathy Patients
Details : Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway. It is being evaluated for the treatment of patients with IgA nephropathy (IgAN).
Brand Name : Fabhalta
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway. It is being evaluated for adults with paroxysmal nocturnal hemoglobinuria (PNH) who have hemolytic anemia.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: Fabhalta
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Novartis' Fabhalta® (iptacopan) Gets Positive CHMP Opinion for PNH
Details : Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway. It is being evaluated for adults with paroxysmal nocturnal hemoglobinuria (PNH) who have hemolytic anemia.
Brand Name : Fabhalta
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LNP023 (iptacopan), an oral, Factor B inhibitor of the alternative complement pathway being developed in Phase 3 for patients with C3 glomerulopathy, which met its primary endpoint by providing clinically meaningful and statistically significant proteinuria reduction.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Nephrology Brand Name: LNP023
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LNP023 (iptacopan), an oral, Factor B inhibitor of the alternative complement pathway being developed in Phase 3 for patients with C3 glomerulopathy, which met its primary endpoint by providing clinically meaningful and statistically significant proteinu...
Brand Name : LNP023
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
FDA approved Fabhalta® (iptacopan), a Factor B inhibitor of the alternative complement pathway 15-17, as the first oral monotherapy for the treatment of adults with paroxysmal nocturnal hemoglobinuria.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: Fabhalta
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 05, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Novartis receives FDA Approval for Fabhalta® (iptacopan), offering Superior Hemoglobin Improvemen...
Details : FDA approved Fabhalta® (iptacopan), a Factor B inhibitor of the alternative complement pathway 15-17, as the first oral monotherapy for the treatment of adults with paroxysmal nocturnal hemoglobinuria.
Brand Name : Fabhalta
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 05, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysis in PNH.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Nephrology Brand Name: LNP023
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysi...
Brand Name : LNP023
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysis in PNH.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: LNP023
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 26, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysi...
Brand Name : LNP023
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 26, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysis in PNH.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: LNP023
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysi...
Brand Name : LNP023
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysis in PNH.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: LNP023
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LNP023 (iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of the alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysi...
Brand Name : LNP023
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LNP023 (Iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysis in PNH.
Lead Product(s): Iptacopan Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: LNP023
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Iptacopan Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LNP023 (Iptacopan) is an investigational first-in-class, orally administered targeted factor B inhibitor of alternative complement pathway. It acts upstream of the C5 terminal pathway, preventing not only intravascular but also extravascular hemolysis in...
Brand Name : LNP023
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 24, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : FABHALTA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 200MG BASE
Packaging :
Approval Date : 2023-12-05
Application Number : 218276
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : FABHALTA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 200MG BASE
Approval Date : 2023-12-05
Application Number : 218276
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]https://www.pharmacompass.com/radio-compass-blog/fda-approvals-slump-19-in-h1-2024-nash-copd-pah-get-new-treatment-options
https://www.pharmacompass.com/pipeline-prospector-blog/pipeline-prospector-aug-2024-otsuka-buys-jnana-lilly-s-market-cap-gains-by-over-us-108-bn-post-new-guidance
Patents & EXCLUSIVITIES
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2028-12-05
Application Number : 218276
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-456
Exclusivity Expiration Date : 2030-12-05
Application Number : 218276
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : I-949
Exclusivity Expiration Date : 2027-08-07
Application Number : 218276
Product Number : 1
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?