
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
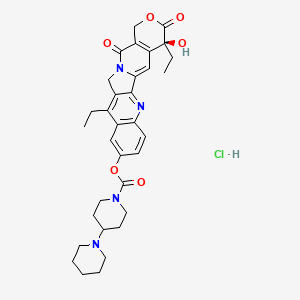

1. 7 Ethyl 10 Hydroxycamptothecin
2. 7-ethyl-10-hydroxycamptothecin
3. Camptosar
4. Camptothecin 11
5. Camptothecin-11
6. Cpt 11
7. Cpt-11
8. Cpt11
9. Irinotecan
10. Irrinotecan
11. Nk012 Compound
12. Sn 38
13. Sn 38 11
14. Sn-38
15. Sn-38-11
16. Sn3811
1. 100286-90-6
2. Irinotecan Hcl
3. Topotecin
4. Campto
5. Camptothecin 11 Hydrochloride
6. Camptosar
7. Cpt 11
8. Cpt-11
9. Irinotecan (hydrochloride)
10. Camptothecin 11
11. U 101440e
12. Chebi:5971
13. (s)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl [1,4'-bipiperidine]-1'-carboxylate Hydrochloride
14. Camptothecin Analog
15. 06x131e4oe
16. 7-ethyl-10-(4-(1-piperidino)-1-piperidino)carbonyloxy Camptothecin Hydrochloride
17. Nsc616348
18. Nsc-616348
19. Ncgc00095190-01
20. Dsstox_cid_25953
21. Dsstox_rid_81249
22. Dsstox_gsid_45953
23. [(19s)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-7-yl] 4-piperidin-1-ylpiperidine-1-carboxylate;hydrochloride
24. Irinotecan Hydrochloride Anhydrous
25. Cas-100286-90-6
26. U-101440e
27. Unii-06x131e4oe
28. Irinotecan Hydrochloride [usan:jan]
29. Mfcd01862255
30. Irinotecanhydrochloride
31. Cpt-11 Hydrochloride
32. Irinotecan Monohydrochloride
33. Schembl4033
34. Camptosar (tn) (pharmacia)
35. (s)-[1,4'-bipiperidine]-1'-carboxylic Acid, 4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl Ester Hydrochloride
36. Spectrum1505821
37. Chembl541887
38. Dtxsid6045953
39. Hms1922j04
40. Pharmakon1600-01505821
41. Amy24895
42. Bcp17234
43. Irinotecan Hydrochloride (anhydrous)
44. Tox21_111479
45. Hy-16562a
46. Nsc759878
47. S5026
48. Akos015901921
49. Tox21_111479_1
50. Ccg-213561
51. Irinotecan Hydrochloride [who-dd]
52. Ncgc00095190-02
53. Ncgc00178697-04
54. (1,4'-bipiperidine)-1'-carboxylic Acid, (4s)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl Ester, Monohydrochloride
55. (1,4'-bipiperidine)-1'-carboxylic Acid, 3,4,12,14-tetrahydro-4,11-diethyl-4-hydroxy-3,4-dioxo-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl Ester, Monohydrochloride, (s)-
56. Ac-28335
57. As-13304
58. I0714
59. Irinotecan Hydrochloride Anhydrous [mi]
60. Irinotecan Hydrochloride, Topoisomerase Inhibitor
61. 286i906
62. A897508
63. Sr-01000763864
64. Q-100016
65. Sr-01000763864-3
66. Q27106952
67. Z1550648758
68. (+)-7-ethyl-10-hydroxycamptothecine 10-(1,4'-bipiperidine)-1'-carboxylate, Monohydrochloride
69. (1,4'-bipiperidine)-1'-carboxylic Acid, (4s)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl Ester, Hydrochloride (1:1)
70. (19s)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaen-7-yl [1,4'-bipiperidine]-1'-carboxylate Hydrochloride
71. (4s)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl [1,4'-bipiperidine]-1'-carboxylic Acid Ester Hydrochloride
72. (4s)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl [1,4'-bipiperidine]-1'-carboxylate Hydrochloride
73. (s)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl[1,4'-bipiperidine]-1'-carboxylatehydrochloride
74. [(19s)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-7-yl] 4-piperidin-1-ylpiperidine-1-carboxylate Hydrochloride
75. [1, 4,11-diethyl-3,4,12, 14-tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano[3',4':6,7] Indolizino[1,2-b]quinolin-9-yl Ester, Monohydrochloride (s)-
76. [1,4'-bipiperidine]-1'-carboxylic Acid,(4s)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl Ester, Monohydrochloride
77. 1-[1-({[(4s)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl]oxy}carbonyl)piperidin-4-yl]piperidin-1-ium Chloride
1. Camptosar
2. Campto
3. Irinotecan
4. Hy-16562
| Molecular Weight | 623.1 g/mol |
|---|---|
| Molecular Formula | C33H39ClN4O6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 622.2558127 g/mol |
| Monoisotopic Mass | 622.2558127 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Irinotecan hydrochloride |
| Drug Label | Irinotecan hydrochloride injection is an antineoplastic agent of the topoisomerase I inhibitor class. Irinotecan hydrochloride was clinically investigated as CPT-11. Irinotecan hydrochloride injection is supplied as a sterile, pale yellow, clear, aqu... |
| Active Ingredient | Irinotecan hydrochloride |
| Dosage Form | Injectable |
| Route | injection; Injection |
| Strength | 20mg/ml; 100mg/5ml (20mg/ml); 500mg/25ml (20mg/ml); 100mg/5ml; 40mg/2ml (20mg/ml) |
| Market Status | Prescription |
| Company | Pliva Lachema; Hospira; Teva Parenteral; Fresenius Kabi Oncol; Accord Hlthcare; Jiangsu Hengrui Med; Hikma Farmaceutica; Teva Pharms Usa; Cipla; Hisun Pharm Hangzhou; Sun Pharma Global; Actavis Elizabeth; Emcure Pharms; Mustafa Nevzat Ilac; Fresenius Kabi |
| 2 of 2 | |
|---|---|
| Drug Name | Irinotecan hydrochloride |
| Drug Label | Irinotecan hydrochloride injection is an antineoplastic agent of the topoisomerase I inhibitor class. Irinotecan hydrochloride was clinically investigated as CPT-11. Irinotecan hydrochloride injection is supplied as a sterile, pale yellow, clear, aqu... |
| Active Ingredient | Irinotecan hydrochloride |
| Dosage Form | Injectable |
| Route | injection; Injection |
| Strength | 20mg/ml; 100mg/5ml (20mg/ml); 500mg/25ml (20mg/ml); 100mg/5ml; 40mg/2ml (20mg/ml) |
| Market Status | Prescription |
| Company | Pliva Lachema; Hospira; Teva Parenteral; Fresenius Kabi Oncol; Accord Hlthcare; Jiangsu Hengrui Med; Hikma Farmaceutica; Teva Pharms Usa; Cipla; Hisun Pharm Hangzhou; Sun Pharma Global; Actavis Elizabeth; Emcure Pharms; Mustafa Nevzat Ilac; Fresenius Kabi |
Topoisomerase I Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE I. (See all compounds classified as Topoisomerase I Inhibitors.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
(S)-8-Ethyl-8-hydroxy-2,3,5,8-tetrahydro-6-oxa-3a-...
CAS Number : 110351-94-5
End Use API : Irinotecan Hydrochloride
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
2’-Amino-5’-hydroxypropiophenone
CAS Number : 35364-15-9
End Use API : Irinotecan Hydrochloride
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
7-Ethyl-10-hydroxycamptothecin (SN-38)
CAS Number : 86639-52-3
End Use API : Irinotecan Hydrochloride
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
2’-Amino-5’-hydroxypropiophenone (AHP)
CAS Number : 35364-15-9
End Use API : Irinotecan Hydrochloride
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
CAS Number : 4897-50-1
End Use API : Irinotecan Hydrochloride
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 4897-50-1
End Use API : Irinotecan Hydrochloride
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
4-Piperidinylpiperidine dihydrochloride
CAS Number : 4876-60-2
End Use API : Irinotecan Hydrochloride
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
4-Piperidinylpiperidine dihydrochloride
CAS Number : 4876-60-2
End Use API : Irinotecan Hydrochloride
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 4897-50-1
End Use API : Irinotecan Hydrochloride
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

CAS Number : 19685-09-7
End Use API : Irinotecan Hydrochloride
About The Company : Chengdu Yazhong Bio-pharmaceutical Co., Ltd., founded in 2002, is a leading company with plant extracts, APIs and medical intermediates. Our company is equipped...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 40MG/2ML (20MG/ML)
Approval Date : 2008-11-21
Application Number : 79068
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 100MG/5ML (20MG/ML)
Approval Date : 2020-11-02
Application Number : 213278
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 100MG/5ML (20MG/ML)
Approval Date : 2010-04-15
Application Number : 78953
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 100MG/5ML (20MG/ML)
Approval Date : 2008-02-27
Application Number : 77915
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AP
Brand Name : CAMPTOSAR
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 40MG/2ML (20MG/ML)
Approval Date : 1996-06-14
Application Number : 20571
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 100MG/5ML (20MG/ML)
Approval Date : 2016-05-03
Application Number : 203380
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 300MG/15ML (20MG/ML)
Approval Date : 2016-05-03
Application Number : 203380
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 40MG/2ML (20MG/ML)
Approval Date : 2008-12-24
Application Number : 78753
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 100MG/5ML (20MG/ML)
Approval Date : 2008-12-24
Application Number : 78753
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : IRINOTECAN HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 40MG/2ML (20MG/ML)
Approval Date : 2011-05-13
Application Number : 90393
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Softgels
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2025-05-02
US Patent Number : 8703181
Drug Substance Claim :
Drug Product Claim :
Application Number : 207793
Patent Use Code : U-1434
Delist Requested :
Patent Use Description : TREATMENT OF PANCREATI...
Patent Expiration Date : 2025-05-02

Patent Expiration Date : 2033-06-12
US Patent Number : 9364473
Drug Substance Claim :
Drug Product Claim :
Application Number : 207793
Patent Use Code : U-1856
Delist Requested :
Patent Use Description : TREATMENT OF METASTATI...
Patent Expiration Date : 2033-06-12

Patent Expiration Date : 2033-06-12
US Patent Number : 10980795
Drug Substance Claim :
Drug Product Claim :
Application Number : 207793
Patent Use Code : U-1848
Delist Requested :
Patent Use Description : TREATMENT OF METASTATI...
Patent Expiration Date : 2033-06-12

Patent Expiration Date : 2033-06-12
US Patent Number : 9339497
Drug Substance Claim :
Drug Product Claim :
Application Number : 207793
Patent Use Code : U-1848
Delist Requested :
Patent Use Description : TREATMENT OF METASTATI...
Patent Expiration Date : 2033-06-12

Patent Expiration Date : 2033-06-12
US Patent Number : 11369597
Drug Substance Claim :
Drug Product Claim :
Application Number : 207793
Patent Use Code : U-1848
Delist Requested :
Patent Use Description : TREATMENT OF METASTATI...
Patent Expiration Date : 2033-06-12

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?