
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Dexferrum
2. Dextran Iron Complex
3. Dextran-iron Complex
4. Dextrofer
5. Feosol
6. Ferridextran
7. Hematran
8. Icar
9. Imfergen
10. Imferon
11. Imperon
12. Imposil
13. Infed
14. Iron Dextran Complex
15. Iron-dextran Complex
16. Norferan
1. 9004-66-4
2. Iron-dextran
3. Sulfuric Acid, Iron Salt
4. Iron;sulfuric Acid
5. Fero Gradumet
6. 10124-49-9
7. Sulfuric Acid, Iron(3+) Salt (3:2)
8. Conferon
9. Sulfuric Acid Iron Salt (1:1)
10. Slow-fe
11. Sulfuric Acid Iron(2+) Salt (1:1)
12. Sulphuric Acid, Iron Salt
13. Wln: Fe S-o4
14. Fe Dextran
15. Sulfuric Acid, Iron Salt (1:?)
16. Einecs 233-336-3
17. Iron Dextran Solution
18. Sulfuric Acid--iron (1/1)
19. Dtxsid80906016
20. Nsc57631
21. Nsc146177
22. S6987
23. Akos030243004
24. Ft-0624534
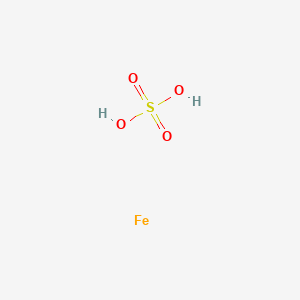
| Molecular Weight | 153.93 g/mol |
|---|---|
| Molecular Formula | FeH2O4S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 153.902315 g/mol |
| Monoisotopic Mass | 153.902315 g/mol |
| Topological Polar Surface Area | 83 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 81.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Hematinics
National Library of Medicine's Medical Subject Headings. Iron Dextran. Online file (MeSH, 2015). Available from, as of May 1, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Iron dextran is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=iron+dextran
Intravenous or intramuscular injections of INFeD are indicated for treatment of patients with documented iron deficiency in whom oral administration is unsatisfactory or impossible. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for INFed (Iron Dextran) Injection (Updated: July 2014). Available from, as of July 21, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abacb7fa-2fc2-471e-9200-944eeac8ca2a
MEDICATION (VET): Nutritional factor (parenteral). Used in iron deficiency anemia, chiefly in pigs.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 948
For more Therapeutic Uses (Complete) data for IRON DEXTRAN (8 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: RISK FOR ANAPHYLACTIC-TYPE REACTIONS. Anaphylactic-type reactions, including fatalities, have followed the parenteral administration of iron dextran injection. Have resuscitation equipment and personnel trained in the detection and treatment of anaphylactic-type reactions readily available during INFeD administration. Administer a test INFeD dose prior to the first therapeutic dose. If no signs or symptoms of anaphylactic-type reactions follow the test dose, administer the full therapeutic INFeD dose. During all INFeD administrations, observe for signs or symptoms of anaphylactic-type reactions. Fatal reactions have followed the test dose of iron dextran injection. Fatal reactions have also occurred in situations where the test dose was tolerated. Use INFeD only in patients in whom clinical and laboratory investigations have established an iron deficient state not amenable to oral iron therapy. Patients with a history of drug allergy or multiple drug allergies may be at increased risk of anaphylactic-type reactions to INFeD.
NIH; DailyMed. Current Medication Information for INFed (Iron Dextran) Injection (Updated: July 2014). Available from, as of July 21, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abacb7fa-2fc2-471e-9200-944eeac8ca2a
Abdominal pain, dyspepsia, nausea, vomiting, diarrhea, metallic taste in the mouth, altered taste, and transient loss of taste perception have occurred in patients receiving iron dextran.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1447
Anaphylactic or anaphylactoid reactions to iron dextran, including fatal anaphylaxis, have been reported. These reactions occur most frequently within the first several minutes of administration and are generally characterized by sudden onset of respiratory difficulty (e.g., wheezing, bronchospasm, rigor, dyspnea, cyanosis), tachycardia, hypotension, respiratory arrest, and/or cardiovascular collapse. The manufacturers state that concomitant use of angiotensin-converting enzyme (ACE) inhibitors may increase the risk for reactions to iron dextran. The level of risk for anaphylactic-type reactions following exposure to specific iron dextran preparations is not known and may vary. Iron dextran preparations differ in chemical characteristics and may differ in clinical effects; the manufacturers state that such preparations are not clinically interchangeable. Acute hypersensitivity reactions to iron dextran have been estimated to occur in 0.2-3% of patients. These reactions have been reported after administration of uneventful test doses of iron dextran as well as after therapeutic doses of the drug. Although it has been suggested that severe systemic reactions, including anaphylactoid reactions, are more common following IV rather than IM administration of iron dextran, the risk of severe systemic reactions following IV or IM administration has not been directly compared and there appears to be no well-substantiated evidence of a difference in the frequency of anaphylactoid reactions following either route of administration.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1447
Large IV doses of iron dextran, such as those used in total-dose infusions, may be associated with an increased frequency of adverse effects, especially delayed (1-2 days) reactions manifested by arthralgia, backache, myalgia, adenopathy, moderate to high fever, backache, chills, dizziness, headache, malaise, nausea, and/or vomiting. The onset of these adverse effects is usually 24-48 hours after administration of the drug, and the effects generally subside within 3-4 days. Delayed adverse effects have also occurred following IM administration and usually subsided within 3-7 days. The etiology of delayed adverse effects is not known, but the symptom complex resembles that of a serum sickness reaction. Patients with rheumatoid arthritis and possibly other inflammatory diseases (e.g., ankylosing spondylitis, lupus erythematosus) may be at particular risk for delayed reactions. IV administration of iron dextran has caused fever and exacerbation or reactivation of joint pain and swelling in patients with rheumatoid arthritis; in addition, exacerbation of ankylosing spondylitis in one patient and arthralgia, myalgia, erythema nodosum, and fever in a patient with lupus erythematosus have been reported. Such exacerbations of underlying inflammatory conditions may respond to nonsteroidal anti-inflammatory agent (NSAIA) therapy and may be prevented with corticosteroid pretreatment.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1447
For more Drug Warnings (Complete) data for IRON DEXTRAN (21 total), please visit the HSDB record page.
For treatment of patients with documented iron deficiency in whom oral administration is unsatisfactory or impossible. Also used to replenish body iron stores in Non-Dialysis Dependent-Chronic Kidney Disease (NDD-CKD) patients receiving or not receiving erythropoietin and in Hemodialysis Dependent (HDD-CKD) and Peritoneal Dialysis Dependent (PDD-CKD) - Chronic Kidney Disease patients receiving an erythropoietin.
Iron dextran is a dark brown, slightly viscous sterile liquid complex of ferric hydroxide and dextran for intravenous or intramuscular use. It is for treatment of patients with documented iron deficiency in whom oral administration is unsatisfactory or impossible. Iron is essential to the formation of hemoglobin and to the function and formation of other heme and nonheme compounds. Untreated depletion of iron stores leads to iron-deficient erythropoiesis and, in turn, to iron deficiency anemia.
Hematinics
Agents which improve the quality of the blood, increasing the hemoglobin level and the number of erythrocytes. They are used in the treatment of anemias. (See all compounds classified as Hematinics.)
Absorption
The major portion of intramuscular injections of iron dextran is absorbed within 72 hours; most of the remaining iron is absorbed over the ensuing 3 to 4 weeks.
Route of Elimination
Dextran, a polyglucose, is either metabolized or excreted.
Following intramuscular administration, iron dextran is absorbed from the site of injection primarily through the lymphatic system. Absorption takes place in two stages. Approximately 60% of the dose or iron dextran inected is absorbed within 72 hours of administration. Ninety percent of the dose is absorbed in the second, slower, phase lasting approximately 1 to 3 weeks in duration. The remaining 10% of the dose is gradually absorbed over a period of several months or longer.
Health Canada; Product Monograph for Dexiron (Iron Dextran Injection, USP (50 mg Elemental Iron/mL) 1 mL, 2 ml Single-Dose Vials), Drug Identification Number (DIN): 02205963 p.16 (Date of Revision: January 2, 2013). Available from, as of July 21, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
Following IM or IV injection, iron dextran is gradually cleared from plasma by reticuloendothelial cells of liver, spleen, and bone marrow. Results of several studies indicate that /a/ variable portion of IV dose... may be stored in unusable form in bone marrow.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1448
In vitro studies have shown that removal of iron dextran by dialysis is negligible.
NIH; DailyMed. Current Medication Information for INFed (Iron Dextran) Injection (Updated: July 2014).
/MILK/ Traces of unmetabolized iron dextran are excreted in human milk.
NIH; DailyMed. Current Medication Information for INFed (Iron Dextran) Injection (Updated: July 2014). Available from, as of July 21, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abacb7fa-2fc2-471e-9200-944eeac8ca2a
For more Absorption, Distribution and Excretion (Complete) data for IRON DEXTRAN (6 total), please visit the HSDB record page.
Dextran, a polyglucose, is either metabolized or excreted.
Dextran, a polyglucose, is either metabolized or excreted. Only traces of unmetabolized iron dextran are excreted in the urine, bile or feces.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1448
5 hours (some indications that it can be as long as 10 hours)
After intravenous injection of 2 mL of DexIron (100 mg iron) to renal dialysis patients ... the average half-life of iron dextran in serum was 58.9 hours, ranging from 9.4 to 87.4 hours in 20 patients.
Health Canada; Product Monograph for Dexiron (Iron Dextran Injection, USP (50 mg Elemental Iron/mL) 1 mL, 2 ml Single-Dose Vials), Drug Identification Number (DIN): 02205963 p.16 (Date of Revision: January 2, 2013). Available from, as of July 21, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
Various studies involving intravenously administered (59)Fe iron dextran to iron deficient subjects, some of whom had coexisting diseases, have yielded half-life values ranging from 5 hours to more than 20 hours. The 5-hour value was determined for (59)Fe iron dextran from a study that used laboratory methods to separate the circulating (59)Fe iron dextran from the transferrin-bound (59)Fe. The 20-hour value reflects a half-life determined by measuring total (59)Fe, both circulating and bound. It should be understood that these half-life values do not represent clearance of iron from the body. Iron is not easily eliminated from the body and accumulation of iron can be toxic.
NIH; DailyMed. Current Medication Information for INFed (Iron Dextran) Injection (Updated: July 2014). Available from, as of July 21, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abacb7fa-2fc2-471e-9200-944eeac8ca2a
After iron dextran is injected, the circulating iron dextran is removed from the plasma by cells of the reticuloendothelial system, which split the complex into its components of iron and dextran. The iron is immediately bound to the available protein moieties to form hemosiderin or ferritin, the physiological forms of iron, or to a lesser extent to transferrin. This iron which is subject to physiological control replenishes hemoglobin and depleted iron stores.
... Circulating iron dextran is removed from the plasma by cells of the reticuloendothelial system, which split the complex into its components of iron and dextran. The iron is immediately bound to the available protein moieties to form hemosiderin or ferritin, the physiological forms of iron, or to a lesser extent to transferrin. This iron which is subject to physiological control replenishes hemoglobin and depleted iron stores.
NIH; DailyMed. Current Medication Information for INFed (Iron Dextran) Injection (Updated: July 2014).
... The toxicity of iron in biological systems is believed to be attributed to its ability to catalyze the generation of oxygen-free radicals. In the current investigation, the dose-dependent effects of chronic iron-loading on heart tissue concentrations of iron, glutathione peroxidase (GPx) activity, free-radical production, and cardiac dysfunction were investigated in a murine model of iron-overload cardiomyopathy. It was shown that chronic iron-overload results in dose-dependent (a) increases in myocardial iron burden, (b) decreases in the protective antioxidant enzyme GPx activity, (c) increased free-radical production, and (d) increased mortality. These findings show that the mechanism of iron-induced heart dysfunction involves in part free radical-mediated processes.
PMID:11232511 Bartfay WJ, Bartfay E; Biol Res Nurs 2 (1): 49-59 (2000)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
67
PharmaCompass offers a list of Iron Dextran API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Iron Dextran manufacturer or Iron Dextran supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Iron Dextran manufacturer or Iron Dextran supplier.
PharmaCompass also assists you with knowing the Iron Dextran API Price utilized in the formulation of products. Iron Dextran API Price is not always fixed or binding as the Iron Dextran Price is obtained through a variety of data sources. The Iron Dextran Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Iron Dextran manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Iron Dextran, including repackagers and relabelers. The FDA regulates Iron Dextran manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Iron Dextran API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Iron Dextran manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Iron Dextran supplier is an individual or a company that provides Iron Dextran active pharmaceutical ingredient (API) or Iron Dextran finished formulations upon request. The Iron Dextran suppliers may include Iron Dextran API manufacturers, exporters, distributors and traders.
click here to find a list of Iron Dextran suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Iron Dextran DMF (Drug Master File) is a document detailing the whole manufacturing process of Iron Dextran active pharmaceutical ingredient (API) in detail. Different forms of Iron Dextran DMFs exist exist since differing nations have different regulations, such as Iron Dextran USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Iron Dextran DMF submitted to regulatory agencies in the US is known as a USDMF. Iron Dextran USDMF includes data on Iron Dextran's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Iron Dextran USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Iron Dextran suppliers with USDMF on PharmaCompass.
A Iron Dextran written confirmation (Iron Dextran WC) is an official document issued by a regulatory agency to a Iron Dextran manufacturer, verifying that the manufacturing facility of a Iron Dextran active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Iron Dextran APIs or Iron Dextran finished pharmaceutical products to another nation, regulatory agencies frequently require a Iron Dextran WC (written confirmation) as part of the regulatory process.
click here to find a list of Iron Dextran suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Iron Dextran as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Iron Dextran API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Iron Dextran as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Iron Dextran and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Iron Dextran NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Iron Dextran suppliers with NDC on PharmaCompass.
Iron Dextran Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Iron Dextran GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Iron Dextran GMP manufacturer or Iron Dextran GMP API supplier for your needs.
A Iron Dextran CoA (Certificate of Analysis) is a formal document that attests to Iron Dextran's compliance with Iron Dextran specifications and serves as a tool for batch-level quality control.
Iron Dextran CoA mostly includes findings from lab analyses of a specific batch. For each Iron Dextran CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Iron Dextran may be tested according to a variety of international standards, such as European Pharmacopoeia (Iron Dextran EP), Iron Dextran JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Iron Dextran USP).
