


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
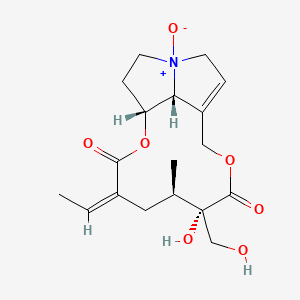

1. Ret-no
2. Retrorsine N-oxide
1. Retrorsine N-oxide
2. 15503-86-3
3. (5r,6s,9a1r,14ar,z)-3-ethylidene-6-hydroxy-6-(hydroxymethyl)-5-methyl-2,7-dioxo-3,4,5,6,7,9,9a1,11,12,13,14,14a-dodecahydro-2h-[1,6]dioxacyclododecino[2,3,4-gh]pyrrolizine 12-oxide
4. [1,6]dioxacyclododecino[2,3,4-gh]pyrrolizine-2,7-dione, 3-ethylidene-3,4,5,6,9,11,13,14,14a,14b-decahydro-6-hydroxy-6-(hydroxymethyl)-5-methyl-, 12-oxide, (3z,5r,6s,14ar,14br)-
5. Chebi:5977
6. Dtxsid4020747
7. Retrorsine-n-oxide , Hplc Grade
8. Zinc4098666
9. Mfcd00074872
10. Hy-116832
11. Cs-0066628
12. Q27106956
13. (3z,5r,6s,14ar,14br)-3-ethylidene-6-hydroxy-6-(hydroxymethyl)-5-methyl-3,4,5,6,9,11,13,14,14a,14b-decahydro[1,6]dioxacyclododecino[2,3,4-gh]pyrrolizine-2,7-dione 12-oxide
| Molecular Weight | 367.4 g/mol |
|---|---|
| Molecular Formula | C18H25NO7 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 367.16310214 g/mol |
| Monoisotopic Mass | 367.16310214 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 673 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Isatidine is not converted into pyrrolic metabolites by isolated rat liver microsomes. It is, However, reduced to retrorsine in the intestinal tract of rats, possibly by the gut flora, thus accounting for the large increase in toxicity when isatidine is administered by mouth rather than parenterally.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 272 (1976)
Levels of pyrrolic metabolites bound to liver tissues and responsible for hepatotoxicity in rats given pyrrolizidine alkaloids did not reflect rates of formation of such metabolites measured in vitro. In animals, additional factors could influence formation and tissue binding of pyrrolic metabolites, including detoxication of alkaloids by hydrolysis and chemical reactivity and stability of toxic metabolites.
PMID:6825198 Mattocks A et al;Chem-Biol Interact 43(2): 209 (1983)


