
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
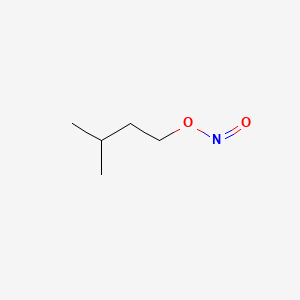

1. Isopentyl Nitrite
1. Isopentyl Nitrite
2. 110-46-3
3. 3-methylbutyl Nitrite
4. Amilnitrite
5. Vaporole
6. Nitrous Acid, 3-methylbutyl Ester
7. 3-methylbutanol Nitrite
8. Aspiral
9. Pentanoli Nitris
10. Nitrous Acid, Isopentyl Ester
11. Amyl Nitrite I
12. Nitramyl (van)
13. Isopentyl Alcohol, Nitrite
14. Amyl Nitrite (van)
15. Amyl Nitrite [usan]
16. Isoamylnitrite
17. Iso-amylnitrite
18. Iso-amyl Nitrite
19. Nsc 7903
20. Vaporole (tn)
21. Aspiral (tn)
22. 5n0u5tuc9z
23. Chebi:2691
24. Nci-c50179
25. Nsc-7903
26. Mfcd00002057
27. Ncgc00091066-01
28. Dsstox_cid_2605
29. Dsstox_rid_77794
30. Dsstox_gsid_25455
31. Amyl Nitrit
32. Cas-110-46-3
33. Ccris 1098
34. Hsdb 606
35. Einecs 203-770-8
36. Unii-5n0u5tuc9z
37. Amyl Nitrite, Mixed Isomers
38. Brn 0969510
39. Isopentylnitrit
40. Amylnitrit
41. Isoamyinitrite
42. Isopentylnitrite
43. Ai3-25183
44. Nitrous Acid 3-methylbutyl Ester
45. Nitrous Acid Isoamyl
46. Vaporole Amyl Nitrite
47. Nitrite Isopentyl Alcohol
48. Isopentyl Nitrite, 96%
49. Wln: Ono2y
50. Amyl Nitrite [jan]
51. Amyl Nitrite(mixed Isomers)
52. 3-methyl-1-nitrosooxybutane
53. Isopentyl Ester Nitrous Acid
54. Amyl Nitrite [hsdb]
55. Schembl23785
56. Amyl Nitrite (jp15/usp)
57. Amyl Nitrite (jp17/usp)
58. Isoamyl Nitrite [mi]
59. 3-methyl-1-nitrosooxy-butane
60. Chembl1535371
61. Dtxsid9025455
62. Amy3781
63. Isoamyl Nitrite 97%, Stabilized
64. Nsc7903
65. Act05198
66. Zinc3830220
67. Tox21_111074
68. Tox21_200983
69. Stl264239
70. Akos009157290
71. Isopentyl Nitrite, >=97.0% (gc)
72. Wt82484
73. Ncgc00091066-02
74. Ncgc00258536-01
75. Ls-13086
76. Db-002757
77. Ft-0627442
78. I0089
79. C07457
80. D00517
81. Sr-01000944365
82. J-802226
83. Sr-01000944365-1
84. Q27888090
85. F0001-0219
86. Isoamyl Nitrite, Stab. With 0.2% Anhyd. Sodium Carbonate
| Molecular Weight | 117.15 g/mol |
|---|---|
| Molecular Formula | C5H11NO2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 117.078978594 g/mol |
| Monoisotopic Mass | 117.078978594 g/mol |
| Topological Polar Surface Area | 38.7 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 63.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...In emergency treatment of cyanide poisoning... for this purpose, sodium nitrite is employed iv, but amyl nitrite may be inhaled while solution of sodium nitrite is being prepared.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
In angina pectoris, pain is eased by vasodilation of coronary arteries produced by amyl nitrite.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
Basic pharmacological action of nitrites is to relax smooth muscle. Relaxation is nonspecific... /nitrites/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 728
Amyl nitrite also has been used to produce changes in the intensity of heart murmurs. Murmurs resulting from stenosis of any of the 4 cardiac valves or from idiopathic hypertrophic subaortic stenosis will become louder after amyl nitrite administration. Murmurs resulting from aortic or mitral regurgitation usually decrease in intensity.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1879
For more Therapeutic Uses (Complete) data for ISOAMYL NITRITE (8 total), please visit the HSDB record page.
The nitrates and nitrites should be used with caution, if at all, in patients with increased intracranial pressure (e.g., head trauma, cerebral hemorrhage) and are contraindicated in patients with severe anemia or with a previous idiosyncratic or hypersensitivity reaction to these drugs. /Nitrates and nitrites/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1876
Since amyl nitrite increases intraocular pressure as well as that of cerebrospinal fluid, it should be used with caution in pt with glaucoma or cerebral hemorrhage.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 24:12
Headache, the most frequent adverse effect may be severe (persistent or transient) and is perceived as a pulsating, throbbing sensation; headache is especially common after inhalation of amyl nitrite. /Nitrates and nitrites/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1875
VET: Causes intense and rapid lowering of blood pressure and increases in heart rate.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 19
For more Drug Warnings (Complete) data for ISOAMYL NITRITE (7 total), please visit the HSDB record page.
Amyl nitrite is readily absorbed via the respiratory tract...
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1878
Approx 1/3 of the inhaled amy nitrite is excreted in the urine.
Medical Economics Co; Physicians Desk Reference- Generics 2nd ed p.212 (1996)
Amyl nitrite ... probably is rapidly hydrolyzed to isoamyl alcohol and nitrite ion.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1878

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
13
PharmaCompass offers a list of Isoamyl Nitrite API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isoamyl Nitrite manufacturer or Isoamyl Nitrite supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isoamyl Nitrite manufacturer or Isoamyl Nitrite supplier.
PharmaCompass also assists you with knowing the Isoamyl Nitrite API Price utilized in the formulation of products. Isoamyl Nitrite API Price is not always fixed or binding as the Isoamyl Nitrite Price is obtained through a variety of data sources. The Isoamyl Nitrite Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Isoamyl Nitrite manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Isoamyl Nitrite, including repackagers and relabelers. The FDA regulates Isoamyl Nitrite manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Isoamyl Nitrite API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Isoamyl Nitrite manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Isoamyl Nitrite supplier is an individual or a company that provides Isoamyl Nitrite active pharmaceutical ingredient (API) or Isoamyl Nitrite finished formulations upon request. The Isoamyl Nitrite suppliers may include Isoamyl Nitrite API manufacturers, exporters, distributors and traders.
click here to find a list of Isoamyl Nitrite suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Isoamyl Nitrite Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Isoamyl Nitrite GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Isoamyl Nitrite GMP manufacturer or Isoamyl Nitrite GMP API supplier for your needs.
A Isoamyl Nitrite CoA (Certificate of Analysis) is a formal document that attests to Isoamyl Nitrite's compliance with Isoamyl Nitrite specifications and serves as a tool for batch-level quality control.
Isoamyl Nitrite CoA mostly includes findings from lab analyses of a specific batch. For each Isoamyl Nitrite CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Isoamyl Nitrite may be tested according to a variety of international standards, such as European Pharmacopoeia (Isoamyl Nitrite EP), Isoamyl Nitrite JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Isoamyl Nitrite USP).
