
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
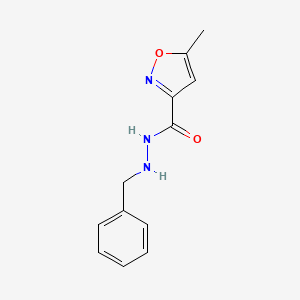

1. 59-63-2
2. Isocarboxazide
3. Marplan
4. Enerzer
5. N'-benzyl-5-methylisoxazole-3-carbohydrazide
6. Isocarbonazid
7. Benazide
8. Isocarbossazide
9. Isocarboxyzid
10. Marplon
11. N'-benzyl-5-methyl-1,2-oxazole-3-carbohydrazide
12. Isocarbossazide [dcit]
13. Maraplan
14. Isocarboxazide [inn-french]
15. Isocarboxazidum [inn-latin]
16. Ro 5-0831
17. Isocarboxazida [inn-spanish]
18. Bmih
19. Ro 5-0831/1
20. 5-methyl-3-isoxazolecarboxylic Acid 2-benzylhydrazide
21. 3-isoxazolecarboxylic Acid, 5-methyl-, 2-(phenylmethyl)hydrazide
22. N'-benzyl N-methyl-5-isoxazolecarboxylhydrazide-3
23. Nsc 169893
24. Isocarboxazid (inn)
25. Nsc-169893
26. 59-63-2 (free Base)
27. Mls003106729
28. 3-isoxazolecarboxylic Acid, 5-methyl-, 2-benzylhydrazide
29. 1-benzyl-2-(5-methyl-3-isoxazolylcarbonyl)hydrazine
30. Cas-59-63-2
31. Ncgc00016267-01
32. Isocarboxazida
33. Isocarboxazidum
34. Ro-5-0831
35. 34237v843t
36. Isocarboxazid [inn]
37. Smr001233334
38. Marplan (tn)
39. Ccris 9178
40. Sr-01000841192
41. Einecs 200-438-4
42. Brn 0201295
43. 1-benzyl-2-(5-methyl-3-isoxazolyl-carbonyl)hydrazine
44. Isocarboxazid [usp:inn:ban]
45. Unii-34237v843t
46. Isocarboxazid (icd)
47. Prestwick0_000795
48. Prestwick1_000795
49. Prestwick2_000795
50. Prestwick3_000795
51. Dsstox_cid_3171
52. Isocarboxazid [mi]
53. Dsstox_rid_76902
54. Dsstox_gsid_23171
55. Schembl49562
56. Bspbio_000930
57. 4-27-00-03999 (beilstein Handbook Reference)
58. Mls002154005
59. Isocarboxazid [mart.]
60. Isocarboxazid(200mg)
61. 5-methyl-n'-(phenylmethyl)isoxazole-3-carbohydrazide
62. Spbio_002869
63. Isocarboxazid [usp-rs]
64. Isocarboxazid [who-dd]
65. Bpbio1_001024
66. Gtpl7204
67. Zinc1587
68. Chembl1201168
69. Dtxsid4023171
70. Wln: T5noj C1 Evmm1r
71. Chebi:93635
72. Isocarboxazid (200 Mg)
73. Bdbm163692
74. Hms1570o12
75. Hms2097o12
76. Hms2230e11
77. Hms3369g18
78. Hms3714o12
79. Hms3887k05
80. Isocarboxazid [orange Book]
81. Tox21_110336
82. Nsc169893
83. Akos016003091
84. Ccg-220795
85. Db01247
86. Ncgc00016267-02
87. Ncgc00016267-03
88. Ncgc00016267-06
89. Ac-24841
90. Hy-13929
91. Ab00513923
92. Cs-0008613
93. Ft-0670438
94. 3-isoxazolecarboxylic Acid, 2-benzylhydrazide
95. D02580
96. N'-benzyl-5-methyl-3-isoxazolecarbohydrazide #
97. A914188
98. Q409595
99. Sr-01000841192-2
100. Sr-01000841192-3
101. 3-(2-benzylhydrazocarboxy)-5-methyl-isoxazole
102. 3-isoxazolecarboxylic Acid, 2-(phenylmethyl)hydrazide
103. 5-methyl-n'-(phenylmethyl)-3-isoxazolecarbohydrazide
104. Brd-k93332168-001-03-2
105. Isocarboxazid 5-methyl-3-isoxazole-carboxylic Acid 2-benzylhydrazide, Aldrichcpr
| Molecular Weight | 231.25 g/mol |
|---|---|
| Molecular Formula | C12H13N3O2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 231.100776666 g/mol |
| Monoisotopic Mass | 231.100776666 g/mol |
| Topological Polar Surface Area | 67.2 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 254 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Marplan |
| PubMed Health | Isocarboxazid (By mouth) |
| Drug Classes | Antidepressant |
| Drug Label | Marplan (isocarboxazid), a monoamine oxidase inhibitor, is available for oral administration in 10-mg tablets. Each tablet also contains lactose, corn starch, povidone, D&C Red No.27, FD&C Yellow No.6, and magnesium stearate. Chemically, isocarbo. |
| Active Ingredient | Isocarboxazid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Validus Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Marplan |
| PubMed Health | Isocarboxazid (By mouth) |
| Drug Classes | Antidepressant |
| Drug Label | Marplan (isocarboxazid), a monoamine oxidase inhibitor, is available for oral administration in 10-mg tablets. Each tablet also contains lactose, corn starch, povidone, D&C Red No.27, FD&C Yellow No.6, and magnesium stearate. Chemically, isocarbo. |
| Active Ingredient | Isocarboxazid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Validus Pharms |
Isocarboxazid is indicated for the treatment of the enduring and debilitating symptoms of depression that have not responded to other antidepressant drugs. Depression is a common but serious mood disorder. The patient will present changes in its feelings, thoughts, and ability to handle everyday activities. For a mood disorder to be considered as depression, the symptoms should be present for at least two weeks.
FDA Label
In vivo and in vitro studies demonstrated isocarboxazid-driven inhibition of MAO in the brain, heart, and liver. The reduced MAO activity, caused by isocarboxazid, results in an increased concentration of serotonin, epinephrine, norepinephrine, and dopamine in storage sites throughout the central nervous system (CNS) and sympathetic nervous system. The increase of one or more monoamines is the basis for the antidepressant activity of MAO inhibitors like isocarboxazid.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Monoamine Oxidase Inhibitors
A chemically heterogeneous group of drugs that have in common the ability to block oxidative deamination of naturally occurring monoamines. (From Gilman, et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed, p414) (See all compounds classified as Monoamine Oxidase Inhibitors.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AF - Monoamine oxidase inhibitors, non-selective
N06AF01 - Isocarboxazid
Absorption
The pharmacokinetic profile of isocarboxazid have not been fully studied but it is suggested that its properties should be fairly similar to the ones of some analogs like phenelzine and tranylcypromine. These drugs are readily absorbed by the GI tract, present a low bioavailability and reach peak concentrations in 1-2 hours.
Route of Elimination
Most of the eliminated dose is found in the urine, accounting for the 42.5% of the administered dose after 24 hours. From this amount, 75% of the renally eliminated drug is in the form of hippuric acid. Another section of the eliminated dose is observed through the intestinal tract and it accounts for 22% of the administered dose after 24 hours.
The pharmacokinetic profile of isocarboxazid have not been fully studied but it is suggested that its properties should be fairly similar to the ones of some analogs like phenelzine and tranylcypromine. These drugs are rapidly metabolized by acetylation in the liver. As part of the metabolism, hippuric acid is a major metabolite.
The pharmacokinetic profile of isocarboxazid have not been fully studied but it is suggested that its properties should be fairly similar to the ones of some analogs like phenelzine and tranylcypromine. The isocarboxazid half-life is of little interest as it is an irreversible monoamine oxidase inhibitor. These drugs present a very short half-life of 1.5-4 hours due to rapid hepatic metabolism.
Isocarboxazid works by irreversibly blocking the action of monoamine oxidases (MAO) in the nervous system. MAO subtypes A and B are involved in the metabolism of serotonin and catecholamine neurotransmitters such as epinephrine, norepinephrine, and dopamine. Isocarboxazid, as a nonselective MAO inhibitor, binds irreversibly to monoamine oxidase-A (MAO-A) and monoamine oxidase-B (MAO-B). Isocarboxacid, like other monoamine oxidase inhibitors, are unique psychopharmacological agents whose clinical effect is related to the direct action of the monoamine oxidases to transform them into reactive metabolites.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
16
PharmaCompass offers a list of Isocarboxazid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isocarboxazid manufacturer or Isocarboxazid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isocarboxazid manufacturer or Isocarboxazid supplier.
PharmaCompass also assists you with knowing the Isocarboxazid API Price utilized in the formulation of products. Isocarboxazid API Price is not always fixed or binding as the Isocarboxazid Price is obtained through a variety of data sources. The Isocarboxazid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Isocarboxazid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Isocarboxazid, including repackagers and relabelers. The FDA regulates Isocarboxazid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Isocarboxazid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Isocarboxazid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Isocarboxazid supplier is an individual or a company that provides Isocarboxazid active pharmaceutical ingredient (API) or Isocarboxazid finished formulations upon request. The Isocarboxazid suppliers may include Isocarboxazid API manufacturers, exporters, distributors and traders.
click here to find a list of Isocarboxazid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Isocarboxazid DMF (Drug Master File) is a document detailing the whole manufacturing process of Isocarboxazid active pharmaceutical ingredient (API) in detail. Different forms of Isocarboxazid DMFs exist exist since differing nations have different regulations, such as Isocarboxazid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Isocarboxazid DMF submitted to regulatory agencies in the US is known as a USDMF. Isocarboxazid USDMF includes data on Isocarboxazid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Isocarboxazid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Isocarboxazid suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Isocarboxazid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Isocarboxazid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Isocarboxazid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Isocarboxazid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Isocarboxazid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Isocarboxazid suppliers with NDC on PharmaCompass.
Isocarboxazid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Isocarboxazid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Isocarboxazid GMP manufacturer or Isocarboxazid GMP API supplier for your needs.
A Isocarboxazid CoA (Certificate of Analysis) is a formal document that attests to Isocarboxazid's compliance with Isocarboxazid specifications and serves as a tool for batch-level quality control.
Isocarboxazid CoA mostly includes findings from lab analyses of a specific batch. For each Isocarboxazid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Isocarboxazid may be tested according to a variety of international standards, such as European Pharmacopoeia (Isocarboxazid EP), Isocarboxazid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Isocarboxazid USP).

