
Synopsis
Synopsis
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
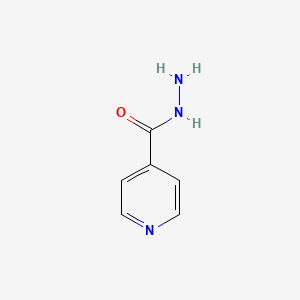

1. Acid Vanillylidenehydrazide, Isonicotinic
2. Ftivazide
3. Hydrazide, Isonicotinic Acid
4. Isonex
5. Isonicotinic Acid Hydrazide
6. Isonicotinic Acid Vanillylidenehydrazide
7. Phthivazid
8. Phthivazide
9. Tubazide
10. Vanillylidenehydrazide, Isonicotinic Acid
1. 54-85-3
2. Isonicotinic Acid Hydrazide
3. Isonicotinohydrazide
4. Pyridine-4-carbohydrazide
5. Isoniazide
6. Nydrazid
7. Rimifon
8. Cotinazin
9. Hydrazid
10. Iscotin
11. Isonicotinic Hydrazide
12. Mybasan
13. Isonicotinylhydrazine
14. Armazid
15. Armazide
16. Atcotibine
17. Dinacrin
18. Ditubin
19. Ertuban
20. Hidrasonil
21. Isidrina
22. Isobicina
23. Isonicotan
24. Laniazid
25. Neumandin
26. Eralon
27. Cedin
28. Hyzyd
29. Andrazide
30. Hydrazide
31. Neoteben
32. Nicizina
33. Nicotibina
34. Nicozide
35. Pycazide
36. Stanozide
37. Tubazide
38. Isolyn
39. Isonex
40. Niconyl
41. Isonicotinhydrazid
42. Antimicina
43. Chemiazid
44. Hidranizil
45. Isocidene
46. Isohydrazide
47. Isonicotil
48. Isonidrin
49. Isonikazid
50. Isonindon
51. Isonizide
52. Isotebezid
53. Pyricidin
54. Pyrizidin
55. Teebaconin
56. Cemidon
57. Chemidon
58. Defonin
59. Diforin
60. Ebidene
61. Eutizon
62. Fimalene
63. Hidrulta
64. Hycozid
65. Idrazil
66. Ismazide
67. Isocotin
68. Isonerit
69. Isonicid
70. Isonico
71. Isonide
72. Isonilex
73. Isonirit
74. Isoniton
75. Isozide
76. Niadrin
77. Nicetal
78. Nidaton
79. Nidrazid
80. Nikozid
81. Nitadon
82. Nyscozid
83. Pelazid
84. Raumanon
85. Retozide
86. Rimicid
87. Rimitsid
88. Tubazid
89. Azuren
90. Evalon
91. Hidrun
92. Inizid
93. Isocid
94. Isonin
95. Isozyd
96. Neoxin
97. Nevin
98. Niplen
99. Antituberkulosum
100. Inah
101. Ido-tebin
102. Tisiodrazida
103. Bacillin
104. Isoniacid
105. Isotinyl
106. Nicazide
107. Nicotibine
108. Nicotisan
109. Phthisen
110. Pyreazid
111. Rimiphone
112. Tubilysin
113. Isotebe
114. Percin
115. Razide
116. Tyvid
117. Isoniazid Sa
118. Neo-tizide
119. 4-pyridinecarboxylic Acid, Hydrazide
120. Gink
121. Armacide
122. Cortinazine
123. Cotinizin
124. Isonicazide
125. Isotamine
126. Robisellin
127. Sauterazid
128. Tibinide
129. Unicozyde
130. Vazadrine
131. Zonazide
132. Dibutin
133. Hyozid
134. Isonicotinoyl Hydrazide
135. Tebecid
136. Tekazin
137. Tubeco
138. Tubicon
139. Tubomel
140. Vederon
141. Zinadon
142. Dow-isoniazid
143. Preparation 6424
144. Isonicotinyl Hydrazide
145. Tb-razide
146. Inh
147. Sanohidrazina
148. Laniozid
149. Niteban
150. Pyridicin
151. Robiselin
152. Roxifen
153. Sauterzid
154. Tebenic
155. Tebexin
156. Tebilon
157. Tibazide
158. Tibison
159. Tibivis
160. Tibizide
161. Tibusan
162. Tuberian
163. Tubizid
164. Unicocyde
165. Tebos
166. Tisin
167. Tizide
168. 4-pyridinecarboxylic Acid Hydrazide
169. Fsr 3
170. Tb-vis
171. Inh-burgthal
172. Tb-phlogin
173. Isoniazidum
174. Zidafimia
175. Isokin
176. Nitebannsc 9659
177. In-73
178. Ru-ef-tb
179. Isonicotinoylhydrazine
180. 4-pyridinecarbonylhydrazine
181. Bp 5015
182. Fsr-3
183. Isonicotinsaeurehydrazid
184. 4-(hydrazinocarbonyl)pyridine
185. 4-pyridinecarbohydrazide
186. 4-pyridylcarbonylhydrazide
187. Isonicotinoylhydrazide
188. Nsc 9659
189. Dianicotyl
190. Ipcazide
191. Hia
192. Rp-5015
193. Pyridine-4-carboxylic Acid Hydrazide
194. Mfcd00006426
195. Isonicotinylhydrazide
196. L 1945
197. 5015 Rp
198. Chebi:6030
199. Isonicotinate Hydrazide
200. 5015 R.p.
201. Nsc-9659
202. Chembl64
203. V83o1voz8l
204. Mls000069444
205. Isozid
206. Nsc9659
207. Cedin (aerosol)
208. Cas-54-85-3
209. Bacillen
210. Tebemid
211. Tubercid
212. Ncgc00016244-09
213. Continazine
214. Tubecotubercid
215. Abdizide
216. Anidrasona
217. Isoniazida
218. Isonizida
219. Smr000059082
220. Tibemid
221. Tibiazide
222. Fetefu
223. Hydra
224. Dsstox_cid_755
225. Dsstox_rid_75771
226. Dsstox_gsid_20755
227. Lanizid
228. 4-pyridinecarboxylic Hydrazide
229. Ry-ef-tb
230. Isoniazidum [inn-latin]
231. Isoniazida [inn-spanish]
232. Azt + Isoniazid
233. Frs-3
234. Laniazid (tn)
235. Ccris 351
236. Usaf Cb-2
237. Hsdb 1647
238. Isonicotinsaeurehydrazid [german]
239. Inhd20
240. Sr-01000003025
241. Einecs 200-214-6
242. Unii-v83o1voz8l
243. Rp 5015
244. Mayambutol
245. Ai3-23936
246. I.a.i.
247. Idrazide Dell'acido Isonicotinico [italian]
248. Isoniazid (inh)
249. Isoniazid/inh
250. Idrazide Dell'acido Isonicotinico
251. Isoniazid [usp:inn:ban:jan]
252. Isonicotinhydrazide
253. Isoniazid(tubizid)
254. Soniazid,(s)
255. Niz
256. Prestwick_578
257. Isoniazid (tubizid)
258. Rifater (salt/mix)
259. Isonicotinic Acid Hydrazide (isoniazid)
260. Isonicotinicacidhydrazide
261. Spectrum_000853
262. Isoniazid [inn]
263. Isoniazid [jan]
264. Opera_id_454
265. Isoniazid [mi]
266. Isonicotinicacid Hydrazide
267. Isoniazid [hsdb]
268. Isonicotinic Acid Hydrazid
269. Prestwick0_000161
270. Prestwick1_000161
271. Prestwick2_000161
272. Prestwick3_000161
273. Spectrum2_000107
274. Spectrum3_000472
275. Spectrum4_000022
276. Spectrum5_000876
277. Isoniazid [vandf]
278. I0138
279. Wln: T6nj Dvmz
280. Isonicotinic Acid Hydrazone
281. Schembl228
282. Biomol-nt_000288
283. Epitope Id:141801
284. Isoniazid [mart.]
285. 4-pyridylcarbonyl Hydrazide
286. Isoniazid [usp-rs]
287. Isoniazid [who-dd]
288. Isoniazid [who-ip]
289. Oprea1_396155
290. Bspbio_000021
291. Bspbio_002204
292. Kbiogr_000423
293. Kbioss_001333
294. Mls001055327
295. Bidd:gt0140
296. Divk1c_000070
297. Spectrum1500355
298. Spbio_000094
299. Spbio_001942
300. Isoniazid (jp17/usp/inn)
301. Bpbio1_000025
302. Bpbio1_001322
303. Schembl2998929
304. Zinc1590
305. Isoniazid [ep Impurity]
306. Isoniazid [orange Book]
307. Component Of Niadox (salt/mix)
308. Dtxsid8020755
309. Isoniazid [ep Monograph]
310. Hms500d12
311. Kbio1_000070
312. Kbio2_001333
313. Kbio2_003901
314. Kbio2_006469
315. Kbio3_001424
316. Isoniazid [usp Monograph]
317. Ninds_000070
318. Rifater Component Isoniazid
319. Hms1568b03
320. Hms1920h09
321. Hms2089i16
322. Hms2091n19
323. Hms2095b03
324. Hms2234g04
325. Hms3259e19
326. Hms3373o01
327. Hms3655l03
328. Hms3712b03
329. Kuc109571n
330. Pharmakon1600-01500355
331. 4-pyridinecarbohydrazide(isoniazid)
332. Isoniazidum [who-ip Latin]
333. Rifamate Component Isoniazid
334. Bcp13791
335. Hy-b0329
336. Str00210
337. [(4-pyridinylcarbonyl)oxy]hydrazine
338. Anti-tnf Monoclonal Antibody & Inh
339. Tox21_113640
340. Tox21_201367
341. Tox21_300193
342. Bbl008409
343. Bdbm50336507
344. Ccg-39710
345. Nsc757078
346. S1937
347. Stk086288
348. Isoniazid Component Of Rifater
349. Akos000119062
350. Isoniazid Component Of Rifamate
351. Isonicotinic Acid Hydrazide(isoniazid)
352. Tox21_113640_1
353. Db00951
354. Ksc-27-048
355. Nc00513
356. Nsc-757078
357. Ps-4129
358. Idi1_000070
359. Upcmld0enat5791176:001
360. Ncgc00016244-01
361. Ncgc00016244-02
362. Ncgc00016244-03
363. Ncgc00016244-04
364. Ncgc00016244-05
365. Ncgc00016244-06
366. Ncgc00016244-07
367. Ncgc00016244-08
368. Ncgc00016244-10
369. Ncgc00016244-11
370. Ncgc00016244-12
371. Ncgc00016244-14
372. Ncgc00016244-15
373. Ncgc00022648-03
374. Ncgc00022648-04
375. Ncgc00022648-05
376. Ncgc00022648-06
377. Ncgc00022648-07
378. Ncgc00254094-01
379. Ncgc00258919-01
380. Isonicotinic Acid Hydrazide [iarc]
381. Sy010614
382. Isoniazid, Vetec(tm) Reagent Grade, 98%
383. Sbi-0051419.p003
384. Ab00052025
385. Bb 0240534
386. Ft-0627424
387. Ft-0670476
388. Isonicotinic Acid Hydrazide [who-ip]
389. Sw196752-3
390. Isonicotinic Acid Hydrazide(isoniazide)
391. C07054
392. D00346
393. D70582
394. Isoniazid, Analytical Standard, >=99% (tlc)
395. Ab00052025-20
396. Ab00052025-21
397. Ab00052025_22
398. Ab00052025_23
399. Ab00052025_24
400. Ab00052025_25
401. A830384
402. Ae-641/02310003
403. Q423169
404. 4-pyridinecarboxylic Acid Hydrazide [who-ip]
405. Sr-01000003025-2
406. Sr-01000003025-3
407. Brd-k87202646-001-26-8
408. Isoniazid, Certified Reference Material, Tracecert(r)
409. Z58981801
410. F0391-0007
411. Isoniazid, European Pharmacopoeia (ep) Reference Standard
412. Isoniazid, United States Pharmacopeia (usp) Reference Standard
413. Isoniazid, Pharmaceutical??secondary??standard;??certified??reference??material
| Molecular Weight | 137.14 g/mol |
|---|---|
| Molecular Formula | C6H7N3O |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 137.058911855 g/mol |
| Monoisotopic Mass | 137.058911855 g/mol |
| Topological Polar Surface Area | 68 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Isoniazid |
| PubMed Health | Isoniazid |
| Drug Classes | Antitubercular |
| Drug Label | Isoniazid is an antibacterial available as 100 mg and 300 mg tablets for oral administration. Each tablet also contains as inactive ingredients: colloidal silicon dioxide, lactose monohydrate, pregelatinized starch, povidone and stearic acid.Isoniazi... |
| Active Ingredient | Isoniazid |
| Dosage Form | Tablet; Syrup; Injectable |
| Route | Injection; Oral |
| Strength | 50mg/5ml; 300mg; 100mg; 100mg/ml |
| Market Status | Prescription |
| Company | Thepharmanetwork; Carolina Medcl; Sandoz; Barr |
| 2 of 6 | |
|---|---|
| Drug Name | Laniazid |
| Active Ingredient | Isoniazid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Lannett |
| 3 of 6 | |
|---|---|
| Drug Name | Rifamate |
| Active Ingredient | rifampin; Isoniazid |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 150mg; 300mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 4 of 6 | |
|---|---|
| Drug Name | Isoniazid |
| PubMed Health | Isoniazid |
| Drug Classes | Antitubercular |
| Drug Label | Isoniazid is an antibacterial available as 100 mg and 300 mg tablets for oral administration. Each tablet also contains as inactive ingredients: colloidal silicon dioxide, lactose monohydrate, pregelatinized starch, povidone and stearic acid.Isoniazi... |
| Active Ingredient | Isoniazid |
| Dosage Form | Tablet; Syrup; Injectable |
| Route | Injection; Oral |
| Strength | 50mg/5ml; 300mg; 100mg; 100mg/ml |
| Market Status | Prescription |
| Company | Thepharmanetwork; Carolina Medcl; Sandoz; Barr |
| 5 of 6 | |
|---|---|
| Drug Name | Laniazid |
| Active Ingredient | Isoniazid |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Lannett |
| 6 of 6 | |
|---|---|
| Drug Name | Rifamate |
| Active Ingredient | rifampin; Isoniazid |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 150mg; 300mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
Antitubercular Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): Antibacterial (tuberculostatic); anti-actinomycotic agent.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 928
Isoniazid is indicated alone in the treatment of latent tuberculosis infection in the following persons: Household members and other close contacts of patients with recently diagnosed tuberculosis who have a positive tuberculin skin test (PPD) of greater than or equal to 5 mm; (tuberculin-negative children and adolescents who have been close contacts of infectious persons within the past 3 months are also candidates for preventative therapy until a repeat PPD is done 12 weeks after contact with the infectious source /NOT included in US product labeling/). /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
Isoniazid is indicated alone in the treatment of latent tuberculosis infection in the following persons: HIV-infected persons of any age with a positive tuberculin skin test of greater than or equal to 5 mm or a past history of a positive tuberculin skin test; also persons with risk factors for HIV infection whose HIV status is unknown but who are suspected of having HIV infection. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
For more Therapeutic Uses (Complete) data for ISONIAZID (16 total), please visit the HSDB record page.
The incidence of and risk factors associated with hepatotoxicity in patients with chronic hepatitis have not been systematically studied. Therefore, we conducted a prospective study that included former drug users who were treated with isoniazid for latent tuberculosis infection. Of 415 patients, 20 (4.8%; 95% confidence interval [CI], 3-7.4) had hepatotoxicity diagnosed, and 6 (1.4%; 95% CI, 0.5-3.2) developed clinical hepatitis, none of whom had serious symptoms. The only 2 factors independently associated with isoniazid hepatotoxicity were excessive alcohol consumption (odds ratio [OR]; 4.2, 95% CI, 1.6-10.8; P=.002) and a high baseline alanine transaminase level (OR, 4.3; 95% CI, 1.6-11.4; P=.002). The presence of hepatitis C virus antibodies was associated with hepatotoxicity only on univariate analysis. Treatment with isoniazid in drug users appears to be safe and well tolerated, although frequent asymptomatic elevations in transaminase levels were observed.
Fernandez-Villar A et al; lin Infect Dis 36 (3): 293-8 (2003)
Mild hepatic dysfunction, as evidenced by mild and transient increases in serum AST (SGOT), ALT (SGPT), and bilirubin concentrations, has occurred in approximately 10-20% of patients receiving isoniazid, usually during the first 4-6 months of therapy. In most cases, enzyme concentrations return to pretreatment values despite continuation of isoniazid, but progressive liver dysfunction, bilirubinuria, jaundice, and severe and sometimes fatal hepatitis have occurred rarely. The incidence of isoniazid-associated hepatitis is lowest in patients younger than 20 years of age and greatest in daily users of alcohol and patients 35 years of age or older. The American Academy of Pediatrics (AAP) states that the incidence of hepatitis during isoniazid therapy is so low in otherwise healthy infants, children, and adolescents that routine determination of serum aminotransferase concentrations are not recommended. The manufacturers state that progressive liver damage may occur in up to 2.3% of patients older than 50 years of age who receive isoniazid. However, data from one study suggest that hepatitis occurs in approximately 4.5% of patients older than 65 years of age who receive the drug. If symptoms of hepatitis or signs suggestive of hepatic damage occur during isoniazid therapy, the drug should be discontinued promptly.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 558
Isoniazid has been reported to cause severe, and sometimes fatal, age related hepatitis. If signs and symptoms of hepatotoxicity occur, isoniazid should be discontinued promptly. The incidence of clinical hepatitis in young, healthy adults is 0.3%, but can increase to 2.6% for those who drink alcohol daily, have chronic liver disease, or are elderly.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1692
Isoniazid may be taken with meals if gastrointestinal irritation occurs. Antacids may also be taken. However, isoniazid should be taken at least 1 hour before aluminum-containing antacids.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1694
For more Drug Warnings (Complete) data for ISONIAZID (30 total), please visit the HSDB record page.
For the treatment of all forms of tuberculosis in which organisms are susceptible.
For active immunisation of chicks from 1 day of age to reduce clinical signs (diarrhoea), intestinal lesions and oocysts output associated with coccidiosis caused by Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria necatrix and Eimeria tenella.
Isoniazid is a bactericidal agent active against organisms of the genus Mycobacterium, specifically M. tuberculosis, M. bovis and M. kansasii. It is a highly specific agent, ineffective against other microorganisms. Isoniazid is bactericidal when mycobacteria grow rapidly and bacteriostatic when they grow slowly.
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
Fatty Acid Synthesis Inhibitors
Compounds that interfere with FATTY ACID SYNTHASE resulting in a reduction of FATTY ACIDS. This is a target mechanism in humans of some ANTINEOPLASTIC AGENTS and ANTI-OBESITY AGENTS and of some ANTI-INFECTIVE AGENTS which interfere with CELL WALL and CELL MEMBRANE formation. (See all compounds classified as Fatty Acid Synthesis Inhibitors.)
QI01AN01
J04AC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AC - Hydrazides
J04AC01 - Isoniazid
Absorption
Readily absorbed following oral administration; however, may undergo significant first pass metabolism. Absorption and bioavailability are reduced when isoniazid is administered with food.
Route of Elimination
From 50 to 70 percent of a dose of isoniazid is excreted in the urine within 24 hours.
ISONIAZID DIFFUSES READILY INTO ALL BODY FLUIDS AND CELLS. THE DRUG IS DETECTABLE IN SIGNIFICANT QUANTITIES IN PLEURAL AND ASCITIC FLUIDS; CONCENTRATIONS IN CEREBROSPINAL FLUID ARE SIMILAR TO THOSE IN THE PLASMA. ISONIAZID PENETRATES WELL INTO CASEOUS MATERIAL. THE CONCENTRATION OF THE AGENT IS INITIALLY HIGHER IN THE PLASMA AND MUSCLE THAN IN THE INFECTED TISSUE, BUT THE LATTER RETAINS THE DRUG FOR A LONG TIME IN QUANTITIES WELL ABOVE THOSE REQUIRED FOR BACTERIOSTASIS.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1157
FROM 75 TO 95% OF A DOSE OF ISONIAZID IS EXCRETED IN THE URINE WITHIN 24 HR, MOSTLY AS METABOLITES.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1157
Readily absorbed following oral administration; however, may undergo significant first pass metabolism. Absorption and bioavailability were reduced when isoniazid was administered with food.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
Widely distributed to all fluids and tissues, including cerebrospinal fluid, pleural and ascitic fluids, skin, sputum, saliva, lungs, muscle, and caseous tissue. Crosses the placenta and is excreted in breast milk.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
For more Absorption, Distribution and Excretion (Complete) data for ISONIAZID (10 total), please visit the HSDB record page.
Primarily hepatic. Isoniazid is acetylated by N -acetyl transferase to N -acetylisoniazid; it is then biotransformed to isonicotinic acid and monoacetylhydrazine. Monoacetylhydrazine is associated with hepatotoxicity via formation of a reactive intermediate metabolite when N-hydroxylated by the cytochrome P450 mixed oxidase system. The rate of acetylation is genetically determined. Slow acetylators are characterized by a relative lack of hepatic N -acetyltransferase.
Isoniazid is inactivated in the liver, mainly by acetylation and dehydrazination. Metabolites of the drug include acetylisoniazid, isonicotinic acid, monoacetylhydrazine, diacetylhydrazine, and isonicotinyl glycine.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 560
/IN MAN/ ... MOST IMPORTANT METABOLITES OF INH IN URINE /WERE FOUND/ TO BE 1-ACETYL-2-ISONICOTINOYLHYDRAZINE (ACETYL INH), N-ACETYL-N'-ISONICOTINIC ACID, ISONICOTINYLGLYCINE, PYRUVIC ACID ISONICOTINYLHYDRAZONE AND ALPHA-OXOGLUTARIC ACID ISONICOTINYLHYDRAZONE ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V4 166 (1974)
IN VIVO METABOLISM OF INH IN RABBIT ... YIELDS ISONICOTINIC ACID AND AMMONIA, LATTER BEING DERIVED FROM RAPID BREAKDOWN OF HYDRAZINE GROUP ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V4 165 (1974)
Acetylation of acetylisoniazid results in the formation of monoacetylhydrazine which has been shown to be a potent hepatotoxin in animals. Microsomal metabolism of monoacetylhydrazine in animals results in production of a reactive acylating species capable of covalently binding with tissue macromolecules (i.e., liver protein) and subsequently causing hepatic necrosis.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 560
For more Metabolism/Metabolites (Complete) data for ISONIAZID (6 total), please visit the HSDB record page.
Isoniazid has known human metabolites that include 3,4,5-Trihydroxy-6-[2-(pyridine-4-carbonyl)hydrazinyl]oxane-2-carboxylic acid and isoniazid N-acetyl.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Fast acetylators: 0.5 to 1.6 hours. Slow acetylators: 2 to 5 hours.
Adults (including elderly patients)- Fast acetylators: 0.5 to 1.6 hr. Slow acetylators: 2 to 5 hr. Acute and chronic liver disease: May be prolonged (6.7 hr vs 3.2 hr in controls),
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
Children (age 1.5 to 15 years)-2.3 to 4.9 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
Neonates-7.8 and 19.8 hours in newborns who received isoniazid transplacentally. The long half-life may be due to the limited acetylation capacity of neonates.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1689
Isoniazid is a prodrug and must be activated by bacterial catalase. Specficially, activation is associated with reduction of the mycobacterial ferric KatG catalase-peroxidase by hydrazine and reaction with oxygen to form an oxyferrous enzyme complex. Once activated, isoniazid inhibits the synthesis of mycoloic acids, an essential component of the bacterial cell wall. At therapeutic levels isoniazid is bacteriocidal against actively growing intracellular and extracellular Mycobacterium tuberculosis organisms. Specifically isoniazid inhibits InhA, the enoyl reductase from Mycobacterium tuberculosis, by forming a covalent adduct with the NAD cofactor. It is the INH-NAD adduct that acts as a slow, tight-binding competitive inhibitor of InhA.
Although the mechanism of action of isoniazid is unknown, several hypotheses have been proposed. These include effects on lipids, nucleic acid biosynthesis, and glycolysis. ... /It has been suggested that/ a primary action of isoniazid /is/ to inhibit the biosynthesis of mycolic acids, important constituents of the mycobacterial cell wall. Because mycolic acids are unique to mycobacteria, this action would explain the high degree of selectivity of the antimicrobial activity of isoniazid. Exposure to isoniazid leads to a loss of acid fastness and a decrease in the quantity of methanol-extractable lipid of the microorganisms.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1157
Isoniazid is bacteriostatic for "resting" bacilli but is bactericidal for rapidly dividing microorganisms. The minimal tuberculostatic concentration is 0.025 to 0.05 ug/ml.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1156

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?