Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
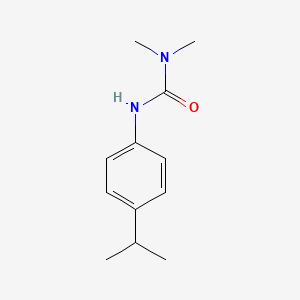

1. Isoproturon-50
2. N'-(4-(1-methylethyl)phenyl)-n,n-dimethylurea
1. 34123-59-6
2. 3-(4-isopropylphenyl)-1,1-dimethylurea
3. Graminon
4. Tolkan
5. Arelon
6. Belgran
7. Arelon R
8. Nocilon
9. Ipu Stefes
10. Ip-flo
11. Alon
12. Hytane 500l
13. Dpx 6774
14. 3-p-cumenyl-1,1-dimethylurea
15. Hoe 16410
16. 1,1-dimethyl-3-(4-propan-2-ylphenyl)urea
17. N-4-isopropylphenyl-n,n-dimethylurea
18. Iprofile
19. Cl 12150
20. Ip 50
21. Urea, 3-p-cumenyl-1,1-dimethyl-
22. Cga-18731
23. N,n-dimethyl-n'-(4-(1-methylethyl)phenyl)urea
24. Urea, 1,1-dimethyl-3-(p-isopropylphenyl)-
25. 3,3-dimethyl-1-[4-(propan-2-yl)phenyl]urea
26. Urea, N,n-dimethyl-n'-[4-(1-methylethyl)phenyl]-
27. Chebi:6049
28. N-(4-isopropylphenyl)-n',n'-dimethylharnstoff
29. Isoproturon 10 Microg/ml In Acetonitrile
30. Isoproturon 100 Microg/ml In Acetonitrile
31. 66066k098p
32. 1,1-dimethyl-3-[4-(propan-2-yl)phenyl]urea
33. Zodiac Tx
34. N,n-dimethyl-n'-[4-(1-methylethyl)phenyl]urea
35. Protugan
36. Isoproturon [bsi:iso]
37. Isoproturon [iso]
38. 1007461-76-8
39. Urea, N,n-dimethyl-n'-(4-(1-methylethyl)phenyl)-
40. Einecs 251-835-4
41. Brn 2214033
42. Tolken
43. Unii-66066k098p
44. N-(isopropyl-4-phenyl)-n',n'-dimethyluree [french]
45. N-(4-isopropylphenyl)-n',n'-dimethylharnstoff [german]
46. Isoproturon [mi]
47. 35689 R.p.
48. N-(isopropyl-4-phenyl)-n',n'-dimethyluree
49. Dsstox_cid_22077
50. Dsstox_rid_79917
51. Dsstox_gsid_42077
52. Schembl62200
53. Ip50
54. Isoproturon, Analytical Standard
55. Chembl2251591
56. Dtxsid1042077
57. Hsdb 6689
58. Zinc392884
59. Hy-b1859
60. Tox21_301329
61. Mfcd00078684
62. 4-isopropylphenyl-n',n'-dimethylurea
63. Akos002959799
64. Gs-3581
65. Isoproturon 100 Microg/ml In Methanol
66. Isoproturon 1000 Microg/ml In Methanol
67. Ncgc00255738-01
68. 1,1-dimethyl-3-(p-isopropylphenyl)-urea
69. N'-(4-isopropylphenyl)-n,n-dimethylurea
70. 3-(4-isopropylphenyl)-1,1-dimethyl-urea
71. Cas-34123-59-6
72. Db-048570
73. Isoproturon 1000 Microg/ml In Acetonitrile
74. N-(4-isopropylphenyl)-n',n'-dimethylurea
75. Cs-0013926
76. Ft-0613679
77. Ft-0670547
78. I0843
79. H10431
80. Isoproturon, Pestanal(r), Analytical Standard
81. A822082
82. J-019459
83. Q2184565
84. 3-(4-isopropylphenyl)-1,1-dimethyl-urea;isoproturon
85. Isoproturon Solution, 100 Ng/mul In Acetonitrile, Pestanal(r), Analytical Standard
| Molecular Weight | 206.28 g/mol |
|---|---|
| Molecular Formula | C12H18N2O |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 206.141913202 g/mol |
| Monoisotopic Mass | 206.141913202 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 206 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Herbicides
Pesticides used to destroy unwanted vegetation, especially various types of weeds, grasses (POACEAE), and woody plants. Some plants develop HERBICIDE RESISTANCE. (See all compounds classified as Herbicides.)
Toxicokinetic behavior and recoveries of isoproturon from feces, urine, and different tissues of goat were determined after 4, 5, 6, and 7 days following single oral administration at 500 mg/kg. Isoproturon was rapidly absorbed and attained blood concentration within 15 min of administration. The kinetic behavior followed a two-compartment open model. The higher half-life (beta) (9.78 +/- 0.33 hr) and V(d)()area (4.49 +/- 0.41 L/kg) associated with lower Cl(B) (0.32 +/- 0.02 L/kg/hr) suggested slow elimination from the blood. Approximately 56% of the total administered compound was recovered from feces. The rate of excretion of isoproturon through feces was maximum at 48 hr and could not be detected beyond 120 hr. The excretion pattern of isoproturon through urine resembled that of feces, and approximately 10-11% was eliminated in urine. A maximum quantity of residue was detected in all tissues of goats slaughtered after 4 days followed by a substantial decline after day 5, and nothing could be detected after day 7. Histopathological study revealed that isoproturon produced moderate cellular changes like fatty degeneration in the liver and kidney and emphysema in the lung after 7 days post administration.
PMID:10554216 Juliet S et al; J Agric Food Chem 46 (1): 178-183 (1998)
Using ring-(14)C-labeled isoproturon (1 ug/L), the uptake into spawn and tadpoles of Bombina bombina and Bombina variegata was investigated. Two percent of the applied radioactivity was found per gram fresh weight in the embryo after 24 hr. Results indicate that the jelly mass of the spawn does not act as a sufficient physical barrier for protection against the uptake and influence of isoproturon (IPU) on the embryo. In vivo metabolism of ring-(14)C-labeled IPU by the cytochrome P-450 system was analyzed in tadpoles. Different metabolites of IPU, such as N-demethylated and C-hydroxylated derivatives, and the olefinic metabolite were detected. In tadpoles of B. variegata, the activity of microsomal and soluble glutathione-S-transferase (sGSTs) toward different model substrates was measured after treatment with IPU. Activities of sGST increased corresponding to elevated stress by IPU dependent on exposure time and dose. Compared to the pure active ingredient IPU, the commercial phenyl-urea herbicide Tolkan Flo, consisting of IPU and an emulsifier, also caused significantly elevated enzymatic response.
PMID:12297088 Greulich K et al; Ecotoxicol Environ Saf 52 (3): 256-66 (2002)
Toxicokinetic behavior and recoveries of isoproturon from feces, urine, and different tissues of goat were determined after 4, 5, 6, and 7 days following single oral administration at 500 mg/kg. ... The higher half-life (beta) (9.78 +/- 0.33 hr) and V(d)()area (4.49 +/- 0.41 L/kg) associated with lower Cl(B) (0.32 +/- 0.02 L/kg/hr) suggested slow elimination from the blood. ...
PMID:10554216 Juliet S et al; J Agric Food Chem 46 (1): 178-183 (1998)
Inhibition of photosynthesis at photosystem II.
Crop Protection Handbook Volume 100, Meister Media Worldwide, Willoughby, OH 2014, p. 370
ABOUT THIS PAGE
76
PharmaCompass offers a list of Isoproturon API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isoproturon manufacturer or Isoproturon supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isoproturon manufacturer or Isoproturon supplier.
PharmaCompass also assists you with knowing the Isoproturon API Price utilized in the formulation of products. Isoproturon API Price is not always fixed or binding as the Isoproturon Price is obtained through a variety of data sources. The Isoproturon Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Isoproturon manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Isoproturon, including repackagers and relabelers. The FDA regulates Isoproturon manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Isoproturon API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Isoproturon supplier is an individual or a company that provides Isoproturon active pharmaceutical ingredient (API) or Isoproturon finished formulations upon request. The Isoproturon suppliers may include Isoproturon API manufacturers, exporters, distributors and traders.
Isoproturon Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Isoproturon GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Isoproturon GMP manufacturer or Isoproturon GMP API supplier for your needs.
A Isoproturon CoA (Certificate of Analysis) is a formal document that attests to Isoproturon's compliance with Isoproturon specifications and serves as a tool for batch-level quality control.
Isoproturon CoA mostly includes findings from lab analyses of a specific batch. For each Isoproturon CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Isoproturon may be tested according to a variety of international standards, such as European Pharmacopoeia (Isoproturon EP), Isoproturon JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Isoproturon USP).
