
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
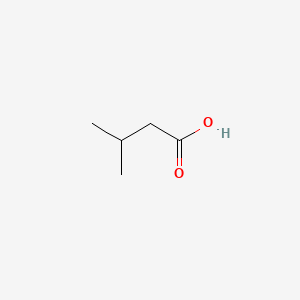

1. 3-methylbutyrate
2. 3-methylbutyric Acid
1. 3-methylbutanoic Acid
2. 503-74-2
3. Isopentanoic Acid
4. 3-methylbutyric Acid
5. Delphinic Acid
6. Butanoic Acid, 3-methyl-
7. Isopropylacetic Acid
8. Isovalerianic Acid
9. Isobutylformic Acid
10. 3-methylbutyrate
11. Beta-methylbutyric Acid
12. Isovalerianic
13. Acetic Acid, Isopropyl-
14. Butyric Acid, 3-methyl-
15. 3-methyl-butanoic Acid
16. Isovalerate
17. Kyselina Isovalerova
18. 3-methyl Butyric Acid
19. 3-methyl-butyric Acid
20. Fema No. 3102
21. 3-methyl-n-butyric Acid
22. Isovaleriansaeure
23. .beta.-methylbutyric Acid
24. Mfcd00002726
25. Nsc 62783
26. 3-methylbuttersaeure
27. B-methylbutyric Acid
28. 3,4-diisovaleryl Adrenaline
29. Chebi:28484
30. 1br7x184l5
31. Butanoic Acid, 3-methyl-, (r)-
32. Isopropylacetate
33. Nsc-62783
34. Iva
35. Isobutyl Formic Acid
36. Isovaleric Acid (natural)
37. Fema Number: 3102
38. Kyselina Isovalerova [czech]
39. Methyl Butanoic Acid
40. Hsdb 629
41. Einecs 207-975-3
42. Methylbutanoic Acid
43. Brn 1098522
44. Unii-1br7x184l5
45. Ai3-24132
46. B-methylbutyrate
47. Iso-valeric Acid
48. 3-methylbutanoicacid
49. Delphinic-acid
50. Iso-c4h9cooh
51. Isovaleric Acid, 99%
52. Dsstox_cid_9182
53. Bmse000373
54. Na 1760 (related)
55. Ec 207-975-3
56. Dsstox_rid_78698
57. Dsstox_gsid_29182
58. Schembl43436
59. Isovaleric Acid [mi]
60. 4-02-00-00895 (beilstein Handbook Reference)
61. Isovaleric Acid [fcc]
62. Methylbutanoic Acid (related)
63. Natural Isovaleric Acid
64. Isopropyl Acetic Acid, Natural
65. Isovaleric Acid [fhfi]
66. Isovaleric Acid [hsdb]
67. Chembl568737
68. Wln: Qv1y1&1
69. Dtxsid5029182
70. Isovaleric Acid [mart.]
71. Isovaleric Acid [who-dd]
72. Zinc388188
73. Amy40214
74. Bcp32116
75. Nsc62783
76. Str08356
77. Isovaleric Acid, Analytical Standard
78. Tox21_201604
79. Bbl027399
80. Lmfa01020181
81. S6287
82. Stl146358
83. Akos000119861
84. Isovaleric Acid, >=99%, Fcc, Fg
85. Cs-w013696
86. Db03750
87. Hy-w012980
88. 3-methylbutyric Acid: Isopropyl-acetate
89. Isovaleric Acid Sodium Salt (salt/mix)
90. Isovaleric Acid, Natural, >=98%, Fg
91. Ncgc00249082-01
92. Ncgc00259153-01
93. 35915-22-1
94. Cas-503-74-2
95. 3-methylbutyric Acid: Isopropyl-acetic Acid
96. Ft-0627533
97. M0182
98. C08262
99. D78213
100. Q415536
101. J-522594
102. F2191-0067
103. Z955123492
104. 3-methylbutanoic Acid;3-methylbutyric Acid;isopentanoic Acid
105. 92634-50-9
| Molecular Weight | 102.13 g/mol |
|---|---|
| Molecular Formula | C5H10O2 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 102.068079557 g/mol |
| Monoisotopic Mass | 102.068079557 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 66.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
It is readily absorbed from the gastrointestinal tract in man.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 3545
In contrast to valeric acid, isovaleric acid is ketogenic. ...Is metabolized by the liver to give two- and three-carbon fragments. The isopropyl fragment of isovaleric acid is readily converted to acetoacetate and is an efficient source of carbon atoms for fatty acid and cholesterol synthesis. Isovaleric acid ... has been identified in the fermentative organs of ruminant animals.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 3545
Isovaleric acid is an intermediate in leucine metabolism. High blood concentrations of isovaleric acid occur in patients with the clinical disorder "isovaleric acidemia." This is a genetic defect of leucine metabolism in which the enzyme isovaleryl-coenzyme A dehydrogenase is inhibited or absent. The disease is characterized by episodic acidosis, slight mental retardation, and an unpleasant body odor.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 3545
RATS RECEIVING LABELED ISOVALERIC ACID EXCRETED NONRADIOACTIVE ISOVALTHINE.
PMID:4225844 KODAMA H ET AL; ACTA MED OKAYAMA 20 (3): 107-13 (1966)
Isovalerate given iv to normal rats was rapidly metabolized, with a 50% decr in plasma concn after 11 min, and this time was shortened to 5 min by the simultaneous admin of glycine. The hypoglycin metabolite methylenecyclopropylacetate-CoA inhibited butyryl-CoA dehydrogenase and isovaleryl-CoA dehydrogenase in liver.
PMID:7317076 Al-Bassam SS, Sherratt HSA; Biochem Pharmacol 30 (20): 2817-24 (1981)
Gas chromatographic and mass spectrometric parameters of isovaleryl-beta-D-glucuronide, a new metabolite in the urine of patients with isovaleric acidemia, were presented. The significance of this metabolite for the detoxication of isovalerate in isovaleric acid media is discussed.
PMID:6652913 Dorland L et al; Clin Chim Acta 134 (1-2): 77-83 (1983)
(14)C-LABELED ISOVALERIC ACID ADMIN ORALLY TO RATS SHOWED ISOPROPYL GROUP WAS MORE EFFICIENTLY UTILIZED FOR CHOLESTEROL SYNTH THAN CARBOXYL GROUPS, & ALSO MORE EFFICIENTLY UTILIZED THAN FATTY ACID SYNTH. CLEAVAGE OF ACID INTO 2 FRAGMENTS OCCURS BEFORE CHOLESTEROL SYNTH. (14)C-LABELED ISOVALERIC ACID ADMIN ORALLY TO RATS APPEARS TO ENHANCE THE INCORPORATION OF CARBON DIOXIDE INTO CHOLESTEROL.
PMID:4225844 KODAMA H ET AL; ACTA MED OKAYAMA 20 (3): 107-13 (1966)
All liver mitochondrial preparations were affected by 1.19 mM isovalerate. Isovaleryl CoA is a potent inhibitor of succinate:CoA ligase (SCL) with positive cooperativity and half-maximal inhibition at 273 +/-11 uM isovaleryl CoA. The investigators suggested that inhibition of the citric acid cycle at the SCL step may be a general mechanism of organic acid toxicity to mitochondria.
PMID:7082321 Bergen BJ et al; Biochem Med 27 (2): 154-60 (1982)
Global Sales Information

ABOUT THIS PAGE
36
PharmaCompass offers a list of Isovaleric Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isovaleric Acid manufacturer or Isovaleric Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isovaleric Acid manufacturer or Isovaleric Acid supplier.
PharmaCompass also assists you with knowing the Isovaleric Acid API Price utilized in the formulation of products. Isovaleric Acid API Price is not always fixed or binding as the Isovaleric Acid Price is obtained through a variety of data sources. The Isovaleric Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Isovaleric Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Isovaleric Acid, including repackagers and relabelers. The FDA regulates Isovaleric Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Isovaleric Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Isovaleric Acid supplier is an individual or a company that provides Isovaleric Acid active pharmaceutical ingredient (API) or Isovaleric Acid finished formulations upon request. The Isovaleric Acid suppliers may include Isovaleric Acid API manufacturers, exporters, distributors and traders.
Isovaleric Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Isovaleric Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Isovaleric Acid GMP manufacturer or Isovaleric Acid GMP API supplier for your needs.
A Isovaleric Acid CoA (Certificate of Analysis) is a formal document that attests to Isovaleric Acid's compliance with Isovaleric Acid specifications and serves as a tool for batch-level quality control.
Isovaleric Acid CoA mostly includes findings from lab analyses of a specific batch. For each Isovaleric Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Isovaleric Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Isovaleric Acid EP), Isovaleric Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Isovaleric Acid USP).
