
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
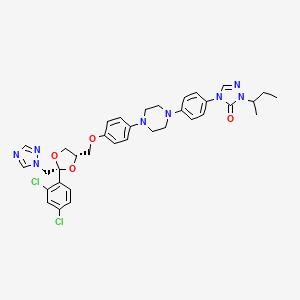

1. Orungal
2. R 51211
3. R-51211
4. R51211
5. Sporanox
1. Sporanox
2. 84625-61-6
3. Oriconazole
4. Itrizole (tn)
5. Sporanox (tn)
6. Itcz
7. Itrizole
8. Itraconazol
9. Orungal
10. Sporonox
11. Itraconazole (sporanox)
12. 2-butan-2-yl-4-[4-[4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one
13. 873066-43-4
14. R-51211
15. Itraconazol [spanish]
16. Itraconazolum [latin]
17. Triasporin
18. Candistat
19. Canditral
20. Itralek
21. Sempera
22. Sporamelt
23. Traconal
24. Chembl22587
25. Itrac
26. Cis-itraconazole
27. Chebi:6076
28. Spherazole Cr
29. Spherazole Ir
30. R51211
31. Nsc-759239
32. Itz
33. Dsstox_cid_3180
34. Dsstox_rid_76908
35. (2r,4s)-itraconazole (mixture Of Diastereomers)
36. Dsstox_gsid_23180
37. Fungitraxx
38. Cladosal 100
39. 2-(butan-2-yl)-4-{4-[4-(4-{[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]phenyl}-2,4-dihydro-3h-1,2,4-triazol-3-one
40. 4-[4-[4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-sec-butyl-1,2,4-triazol-3-one
41. Cas-84625-61-6
42. 304nug5gf4
43. Intraconazole
44. Itraconazolo
45. Sporanox(tm)
46. Ncgc00018268-03
47. 3h-1,2,4-triazol-3-one, 4-(4-(4-(4-((2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-1-piperazinyl)phenyl)-2,4-dihydro-2-(1-methylpropyl)-
48. 4-(4-{4-[4-({[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methyl}oxy)phenyl]piperazin-1-yl}phenyl)-2-(1-methylpropyl)-2,4-dihydro-3h-1,2,4-triazol-3-one
49. 84604-65-9
50. Itraconazole & Nyotran
51. R 51,211
52. Itraconazole [mi]
53. Itraconazole [inn]
54. Itraconazole [jan]
55. Itraconazole [inci]
56. Itraconazole [usan]
57. Itraconazole (jp17/usp)
58. Itraconazole [vandf]
59. Schembl23934
60. Itraconazole [mart.]
61. Mls006011958
62. Itraconazole [usp-rs]
63. Itraconazole [who-dd]
64. Amy922
65. Dtxsid3023180
66. Gtpl11426
67. Itraconazole [green Book]
68. Itraconazole [ep Impurity]
69. Itraconazole [orange Book]
70. Pharmakon1600-01505756
71. Itraconazole [ep Monograph]
72. 4-(4-(4-(4-((2-((1h-1,2,4-triazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2-(sec-butyl)-2,4-dihydro-3h-1,2,4-triazol-3-one
73. Itraconazole [usp Monograph]
74. Tox21_110854
75. Ac-542
76. Bdbm50127138
77. Nsc759239
78. S2476
79. Akos015842738
80. Akos015961385
81. Tox21_110854_1
82. Ccg-270391
83. Db01167
84. Ks-1268
85. Ncgc00274068-01
86. Ncgc00274068-02
87. Ncgc00274068-07
88. 1-(butan-2-yl)-4-{4-[4-(4-{[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]phenyl}-4,5-dihydro-1h-1,2,4-triazol-5-one
89. 4-(4-(4-(4-(((2r,4s)-2-((1h-1,2,4-triazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2-(sec-butyl)-2,4-dihydro-3h-1,2,4-triazol-3-one
90. Hy-17514
91. Smr001827898
92. Itraconazole & Nyotran(liposomal Nystatin)
93. Sbi-0206914.p001
94. Itraconazole (ema Epar: Veterinary)
95. Sw219756-1
96. Itraconazole 2.0 Mg/ml In Dimethyl Sulfoxide
97. D00350
98. Suba-itraconazole Component Itraconazole
99. Ab01274818-01
100. Ab01274818_02
101. Ab01274818_03
102. A933954
103. Itraconazole Component Of Suba-itraconazole
104. Q411229
105. Brd-a23067620-001-01-7
106. (+/-)-1-sec-butyl-4-(p-(4-(p-(((2r*,4s*)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-1-piperazinyl)phenyl)-.delta.(sup 2)-1,2,4-triazolin-5-one
107. 3h-1,2,4-triazol-3-one, 4-[4-[4-[4-[[2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-pipera-zinyl]phenyl]-2,4-dihydro-2-(1-methylpropyl)
108. 4-[4-[4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazole-1-ylmethyl)-1,3-dioxolane-4-yl]methoxy]phenyl]piperazino]phenyl]-2-(1-methylpropyl)-4h-1,2,4-triazole-3(2h)-one
| Molecular Weight | 705.6 g/mol |
|---|---|
| Molecular Formula | C35H38Cl2N8O4 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 11 |
| Exact Mass | 704.2393071 g/mol |
| Monoisotopic Mass | 704.2393071 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 49 |
| Formal Charge | 0 |
| Complexity | 1120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Itraconazole |
| PubMed Health | Itraconazole (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | SPORANOX is the brand name for itraconazole, a synthetic triazole antifungal agent. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It may be represented by the followi... |
| Active Ingredient | Itraconazole |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Sandoz |
| 2 of 4 | |
|---|---|
| Drug Name | Sporanox |
| PubMed Health | Itraconazole (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | SPORANOX is the brand name for itraconazole, a synthetic triazole antifungal agent. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It may be represented by the followi... |
| Active Ingredient | Itraconazole |
| Dosage Form | Capsule; Solution |
| Route | oral; Oral |
| Strength | 10mg/ml; 100mg |
| Market Status | Prescription |
| Company | Janssen Pharms; Janssen Pharma |
| 3 of 4 | |
|---|---|
| Drug Name | Itraconazole |
| PubMed Health | Itraconazole (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | SPORANOX is the brand name for itraconazole, a synthetic triazole antifungal agent. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It may be represented by the followi... |
| Active Ingredient | Itraconazole |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Sandoz |
| 4 of 4 | |
|---|---|
| Drug Name | Sporanox |
| PubMed Health | Itraconazole (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | SPORANOX is the brand name for itraconazole, a synthetic triazole antifungal agent. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It may be represented by the followi... |
| Active Ingredient | Itraconazole |
| Dosage Form | Capsule; Solution |
| Route | oral; Oral |
| Strength | 10mg/ml; 100mg |
| Market Status | Prescription |
| Company | Janssen Pharms; Janssen Pharma |
Antifungal Agents; Antiprotozoal Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Itraconazole capsules are indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: Blastomycosis, pulmonary and extrapulmonary; Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis and Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
Itraconazole capsules are also indicated for the treatment of the following fungal infections in non-immunocompromised patients: Onychomycosis of the toenail, with or without fingernail involvement, due to dermatophytes (tinea unguium) and Onychomycosis of the fingernail due to dermatophytes (tinea unguium). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
/BOXED WARNING/ Congestive Heart Failure, Cardiac Effects: Itraconazole capsules should not be administered for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. If signs or symptoms of congestive heart failure occur during administration of itraconazole capsules, discontinue administration. When itraconazole was administered intravenously to dogs and healthy human volunteers, negative inotropic effects were seen.
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (Updated: June 2014). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d343050e-00bb-440c-a24b-7cd18c97e184
/BOXED WARNING/ Drug Interactions: Coadministration of the following drugs are contraindicated with itraconazole capsules: methadone, disopyramide, dofetilide, dronedarone, quinidine, ergot alkaloids (such as dihydroergotamine, ergometrine (ergonovine), ergotamine, methylergometrine (methylergonovine)), irinotecan, lurasidone, oral midazolam, pimozide, triazolam, felodipine, nisoldipine, ranolazine, eplerenone, cisapride, lovastatin, simvastatin and, in subjects with renal or hepatic impairment, colchicine. Coadministration with itraconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia.
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (Updated: June 2014). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d343050e-00bb-440c-a24b-7cd18c97e184
Itraconazole is contraindicated in patients with known hypersensitivity to the drug or any ingredient in the formulation. Although information concerning cross-sensitivity between itraconazole and other triazole or imidazole antifungal agents is not available, the manufacturer states that itraconazole should be used with caution in individuals hypersensitive to other azoles.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 531
Adverse GI effects have been reported in about 1-11% of patients receiving IV or oral itraconazole for the treatment of systemic fungal infections or oropharyngeal or esophageal candidiasis or for empiric anti-fungal therapy. These adverse GI effects usually are transient and respond to symptomatic treatment without alteration of itraconazole therapy; however, reduction of dosage or discontinuance of the drug occasionally may be required.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 530
For more Drug Warnings (Complete) data for Itraconazole (27 total), please visit the HSDB record page.
For the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: pulmonary and extrapulmonary blastomycosis, histoplasmosis, aspergillosis, and onychomycosis.
FDA Label
For the treatment of aspergillosis and candidiasis in companion birds
Itraconazole is an imidazole/triazole type antifungal agent. Itraconazole is a highly selective inhibitor of fungal cytochrome P-450 sterol C-14 α-demethylation via the inhibition of the enzyme cytochrome P450 14α-demethylase. This enzyme converts lanosterol to ergosterol, and is required in fungal cell wall synthesis. The subsequent loss of normal sterols correlates with the accumulation of 14 α-methyl sterols in fungi and may be partly responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition. Itraconazole exhibits in vitro activity against Cryptococcus neoformans and Candida spp. Fungistatic activity has also been demonstrated in normal and immunocompromised animal models for systemic and intracranial fungal infections due to Cryptococcus neoformans and for systemic infections due to Candida albicans.
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
QJ02AC02
J02AC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AC - Triazole and tetrazole derivatives
J02AC02 - Itraconazole
Absorption
The absolute oral bioavailability of itraconazole is 55%, and is maximal when taken with a full meal.
Route of Elimination
Itraconazole is metabolized predominately by the cytochrome P450 3A4 isoenzyme system (CYP3A4) in the liver, resulting in the formation of several metabolites, including hydroxyitraconazole, the major metabolite. Fecal excretion of the parent drug varies between 3-18% of the dose. Renal excretion of the parent drug is less than 0.03% of the dose. About 40% of the dose is excreted as inactive metabolites in the urine. No single excreted metabolite represents more than 5% of a dose.
Volume of Distribution
796 185 L
Clearance
381 +/- 95 mL/minute [IV administration]
The pharmacokinetics of itraconazole after intravenous administration and its absolute oral bioavailability from an oral solution were studied in a randomized crossover study in 6 healthy male volunteers. The observed absolute oral bioavailability of itraconazole was 55%.
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
The oral bioavailability of itraconazole is maximal when itraconazole capsules are taken with a full meal. The pharmacokinetics of itraconazole were studied in 6 healthy male volunteers who received, in a crossover design, single 100 mg doses of itraconazole as a polyethylene glycol capsule, with or without a full meal. The same 6 volunteers also received 50 mg or 200 mg with a full meal in a crossover design. In this study, only itraconazole plasma concentrations were measured. The respective pharmacokinetic parameters for itraconazole are presented in the table /provided/.
Table: Oral Bioavailability of Itraconazole (Itraconazole capsules): [Table#7591]
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
[Table#7591]
Steady-state concentrations were reached within 15 days following oral doses of 50 mg to 400 mg daily. Values given in the table below are data at steady-state from a pharmacokinetics study in which 27 healthy male volunteers took 200 mg itraconazole capsules b.i.d. (with a full meal) for 15 days [Table#7592]
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
[Table#7592]
Thirty healthy men received single 200 mg doses of itraconazole capsules under fasted conditions either 1) with water; 2) with water, after ranitidine 150 mg b.i.d. for 3 days; or 3) with cola, after ranitidine 150 mg b.i.d. for 3 days. When itraconazole capsules were administered after ranitidine pretreatment, itraconazole was absorbed to a lesser extent than when itraconazole capsules were administered alone, with decreases in AUC0-24 and Cmax of 39% +/- 37% and 42% +/- 39%, respectively. When itraconazole capsules were administered with cola after ranitidine pretreatment, itraconazole absorption was comparable to that observed when itraconazole capsules were administered alone.
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
For more Absorption, Distribution and Excretion (Complete) data for Itraconazole (11 total), please visit the HSDB record page.
Itraconazole is extensively metabolized by the liver into a large number of metabolites, including hydroxyitraconazole, the major metabolite. The main metabolic pathways are oxidative scission of the dioxolane ring, aliphatic oxidation at the 1-methylpropyl substituent, N-dealkylation of this 1-methylpropyl substituent, oxidative degradation of the piperazine ring and triazolone scission.
Itraconazole is metabolized predominantly by the cytochrome P450 3A4 isoenzyme system (CYP3A4), resulting in the formation of several metabolites, including hydroxyitraconazole, the major metabolite. Results of a pharmacokinetics study suggest that itraconazole may undergo saturable metabolism with multiple dosing.
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
Itraconazole (ITZ) is metabolized in vitro to three inhibitory metabolites: hydroxy-itraconazole (OH-ITZ), keto-itraconazole (keto-ITZ), and N-desalkyl-itraconazole (ND-ITZ). The goal of this study was to determine the contribution of these metabolites to drug-drug interactions caused by ITZ. Six healthy volunteers received 100 mg ITZ orally for 7 days, and pharmacokinetic analysis was conducted at days 1 and 7 of the study. The extent of CYP3A4 inhibition by ITZ and its metabolites was predicted using this data. ITZ, OH-ITZ, keto-ITZ, and ND-ITZ were detected in plasma samples of all volunteers. A 3.9-fold decrease in the hepatic intrinsic clearance of a CYP3A4 substrate was predicted using the average unbound steady-state concentrations (C(ss,ave,u)) and liver microsomal inhibition constants for ITZ, OH-ITZ, keto-ITZ, and ND-ITZ. Accounting for circulating metabolites of ITZ significantly improved the in vitro to in vivo extrapolation of CYP3A4 inhibition compared to a consideration of ITZ exposure alone.
PMID:17495874 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3488349 Templeton IE et al; Clin Pharmacol Ther 83 (1): 77-85 (2008)
21 hours
Itraconazole interacts with 14-α demethylase, a cytochrome P-450 enzyme necessary to convert lanosterol to ergosterol. As ergosterol is an essential component of the fungal cell membrane, inhibition of its synthesis results in increased cellular permeability causing leakage of cellular contents. Itraconazole may also inhibit endogenous respiration, interact with membrane phospholipids, inhibit the transformation of yeasts to mycelial forms, inhibit purine uptake, and impair triglyceride and/or phospholipid biosynthesis.
In vitro studies have demonstrated that itraconazole inhibits the cytochrome P450-dependent synthesis of ergosterol, which is a vital component of fungal cell membranes.
US Natl Inst Health; DailyMed. Current Medication Information for Itraconazole (itraconazole) capsule (February 2010). Available from, as of September 27, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16279
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

NDC Package Code : 58032-0110
Start Marketing Date : 2017-11-27
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27410
Submission : 2013-08-01
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-02-08
Pay. Date : 2017-12-28
DMF Number : 12697
Submission : 1997-10-16
Status : Active
Type : II

Certificate Number : R1-CEP 1999-196 - Rev 04
Issue Date : 2021-07-29
Type : Chemical
Substance Number : 1335
Status : Valid

NDC Package Code : 22568-1108
Start Marketing Date : 2021-06-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Dosage Form : Suspension, Tablet
Grade : Oral
Category : Rheology Modifiers, Thickeners and Stabilizers
Dosage Form : Cream / Lotion / Ointment, Gel, Suspension, Tablet
Grade : Oral, Topical
Category : Rheology Modifiers, Thickeners and Stabilizers
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Tablet, Emulsion, Suspension
Grade : Oral
Category : Co-Processed Excipients, Fillers, Diluents & Binders, Rheology Modifiers
Application : Co-Processed Excipients, Fillers, Diluents & Binders, Rheology Modifiers
Excipient Details : HiCel MCG is use in oral suspensions as a stabilizer. It is a good binder for tablets & excellent thickener as well.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Carboxymethyl Cellulose Sodium
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
11
PharmaCompass offers a list of Itraconazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Itraconazole manufacturer or Itraconazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Itraconazole manufacturer or Itraconazole supplier.
PharmaCompass also assists you with knowing the Itraconazole API Price utilized in the formulation of products. Itraconazole API Price is not always fixed or binding as the Itraconazole Price is obtained through a variety of data sources. The Itraconazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Itraconazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Itraconazole, including repackagers and relabelers. The FDA regulates Itraconazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Itraconazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Itraconazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Itraconazole supplier is an individual or a company that provides Itraconazole active pharmaceutical ingredient (API) or Itraconazole finished formulations upon request. The Itraconazole suppliers may include Itraconazole API manufacturers, exporters, distributors and traders.
click here to find a list of Itraconazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Itraconazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Itraconazole active pharmaceutical ingredient (API) in detail. Different forms of Itraconazole DMFs exist exist since differing nations have different regulations, such as Itraconazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Itraconazole DMF submitted to regulatory agencies in the US is known as a USDMF. Itraconazole USDMF includes data on Itraconazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Itraconazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Itraconazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Itraconazole Drug Master File in Japan (Itraconazole JDMF) empowers Itraconazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Itraconazole JDMF during the approval evaluation for pharmaceutical products. At the time of Itraconazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Itraconazole suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Itraconazole Drug Master File in Korea (Itraconazole KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Itraconazole. The MFDS reviews the Itraconazole KDMF as part of the drug registration process and uses the information provided in the Itraconazole KDMF to evaluate the safety and efficacy of the drug.
After submitting a Itraconazole KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Itraconazole API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Itraconazole suppliers with KDMF on PharmaCompass.
A Itraconazole CEP of the European Pharmacopoeia monograph is often referred to as a Itraconazole Certificate of Suitability (COS). The purpose of a Itraconazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Itraconazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Itraconazole to their clients by showing that a Itraconazole CEP has been issued for it. The manufacturer submits a Itraconazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Itraconazole CEP holder for the record. Additionally, the data presented in the Itraconazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Itraconazole DMF.
A Itraconazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Itraconazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Itraconazole suppliers with CEP (COS) on PharmaCompass.
A Itraconazole written confirmation (Itraconazole WC) is an official document issued by a regulatory agency to a Itraconazole manufacturer, verifying that the manufacturing facility of a Itraconazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Itraconazole APIs or Itraconazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Itraconazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Itraconazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Itraconazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Itraconazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Itraconazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Itraconazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Itraconazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Itraconazole suppliers with NDC on PharmaCompass.
Itraconazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Itraconazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Itraconazole GMP manufacturer or Itraconazole GMP API supplier for your needs.
A Itraconazole CoA (Certificate of Analysis) is a formal document that attests to Itraconazole's compliance with Itraconazole specifications and serves as a tool for batch-level quality control.
Itraconazole CoA mostly includes findings from lab analyses of a specific batch. For each Itraconazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Itraconazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Itraconazole EP), Itraconazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Itraconazole USP).

