
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
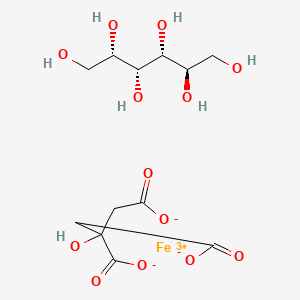

1. Citric Acid -iron -sorbitol
2. Citric Acid, Iron, Sorbitol Drug Combination
3. Iron Sorbitex
4. Iron Sorbitol
5. Iron Sorbitol Citric Acid Complex
6. Yectofer
1. Iron Sorbitex
2. Iron Sorbitex.
3. 1338-16-5
4. 62765-90-6
5. Iron Sorbitol Citrate
6. Yectofer
7. Astra 1572
8. (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol;2-hydroxypropane-1,2,3-tricarboxylate;iron(3+)
9. Hsdb 1967
10. Iron Sorbitex [usan:usp]
11. Iron-sorbitol
12. Schembl60075
13. Dtxsid40928274
14. Citric Acid, Iron, Sorbitol Drug Combination
15. Iron(3+) 2-hydroxypropane-1,2,3-tricarboxylate--hexitol (1/1/1)
| Molecular Weight | 427.12 g/mol |
|---|---|
| Molecular Formula | C12H19FeO13 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 7 |
| Exact Mass | 427.017501 g/mol |
| Monoisotopic Mass | 427.017501 g/mol |
| Topological Polar Surface Area | 262 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 315 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
MEDICATION (VET): A complex of iron, citric acid and sorbitol stabilized with dextrin. Use: im prophylaxis of baby pig anemias indicated it to be inferior to iron dextrans based on Fe blood and tissue levels.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 290
...A total number of 60 pregnant women with the gestational age of 12-34 weeks were included in the study who were suffering from iron deficiency anemia. They were divided in 3 groups (A, B and C). Group A (n=15) received iv iron sucrose according to recommended dose containing 500 mg of iron sucrose for storage, in group B (n=20) iron sucrose was administered according to deficit calculated as per formula but 200 mg of iron was given for storage instead of 500 mg, to reduce cost. While group C received intra muscular iron Sorbitol in the dose used as practice. RESULTS: Mean hemoglobin in group A and B was 8.0 +/- 1.1 g/dL and 8.9 +/- 0.7 respectively, in group C, it was 8.8 +/- 0.9 g/dL. In group A & B initial hemoglobin was assessed 3 weeks post therapy which showed an average rise of 2.8 g/dL (group A) and 1.9 g/dL (group B) and second assessment of Hemoglobin was done prior to delivery (ave: 6.6 weeks) showed a total rise of 3.8 g/dL (group A) and 2.4 g/dL (group B). Pre delivery mean Hemoglobin in group A and B was 11.8 g/dL and 11.3 g/dL respectively. In group C, the Hemoglobin was assessed only prior to delivery (average: 8.4 weeks from the start of therapy), and a rise of 1.4 g/dL was observed with pre delivery mean Hemoglobin of 10.2 g/dL. Target hemoglobin levels i.e. 11 g/dL were achieved by 80% in Group A, 70% in Group B and 28% in Group C by the time of delivery. Blood transfusion was not required in any group. In group A and B one patient had moderate abdominal pain, 2 had weakness and shivering and 3 had phlebitis at the site where intravenous canula was retained. None of patient discontinued the therapy due to any adverse effect. In group C majority complained of pain at injection site while 5 patients dropped out from the study due to intolerance.
PMID:12532571 Wali A et al; J Pak Med Assoc 52 (9): 392-395 (2002)
...Young BALB/CJ mice, from 5-23 days of age, and adult (60-100 days old) mice, some of which were iron deficient due to a low iron diet from the time of weaning, were given iron sc (iron sorbitol, Fe+3, 1.2 mg iron/100g bw/injection). Iron was given as one single dose and the animals killed 20-24 hr later. Control mice were similarly treated with saline. In young mice, from the age 10 days to weaning at 20 days, the serum immunoreactive erythroprotien level, measured 20-24 hr after a single sc dose of iron, was 5 times greater than that in control mice (P<0.0001). In contrast, there was no change in serum immunoreactive erythroprotien 20-24 hr after a single sc dose of iron in adult mice, whether iron-deficient anemic or normal. Thus, during the "early anemia" in young mice over 10 days old, iron increased serum immunoreactive erythroprotien levels, whereas it had no such effect in iron-deficient and normal adult mice.
Bechensteen AG, Halvorsen S; Br J Hamatol 94 (3): 529-532 (1996)
/Iron sorbitol-citric acid complex/ ... is a parenteral form of medication (for IM injection) used in iron deficiency anemia in humans.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V2 164 (1973)
The preparation... does not produce hemolysis /and/ affects coagulation only at very high concentrations.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 917
Determinations of hemoglobin concentration should be conducted periodically during... therapy...and administration of drug should be discontinued when hemoglobin concentration reaches a normal level, even if calculated dosage has not been given.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 20:0404
Iron sorbitex should not be administered to patients with low unsaturated iron binding capacities.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 20:0404
Drug is not currently recommended for use in infants or children.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 20:0404
For more Drug Warnings (Complete) data for IRON SORBITOL CITRATE (12 total), please visit the HSDB record page.
Iron sorbitol is absorbed directly into the bloodstream as well as via the lymphatic system. Sixty-six percent of the intramuscular injection is absorbed within 3 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1672
Iron sorbitex appears to be absorbed directly into the blood stream as well as through the lymphatic system. In man, serum iron content rises rapidly, reaching a peak about 2 hr after im administration; the iron is rapidly cleared from the serum, and bone marrow uptake occurs within 24-48 hr.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 20:0404
Following im injection of 1.4-2.0 mL (59)Fe-labelled iron sorbitol-citric acid complex containing 70-100 mg iron to 12 human patients with iron deficiency anemia or sideropenia ... disappearance of radioactivity from injection site /occurred, no significant amount/...detected at 10 hr after injection. 33% of dose was excreted in urine and <1% in feces.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V2 172 (1973)
30% of the iron excreted in urine in 24 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1673
For more Absorption, Distribution and Excretion (Complete) data for IRON SORBITOL CITRATE (9 total), please visit the HSDB record page.
Market Place

ABOUT THIS PAGE
15
PharmaCompass offers a list of Iron Sorbitol Citrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Iron Sorbitol Citrate manufacturer or Iron Sorbitol Citrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Iron Sorbitol Citrate manufacturer or Iron Sorbitol Citrate supplier.
PharmaCompass also assists you with knowing the Iron Sorbitol Citrate API Price utilized in the formulation of products. Iron Sorbitol Citrate API Price is not always fixed or binding as the Iron Sorbitol Citrate Price is obtained through a variety of data sources. The Iron Sorbitol Citrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Jectofer manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Jectofer, including repackagers and relabelers. The FDA regulates Jectofer manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Jectofer API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Jectofer manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Jectofer supplier is an individual or a company that provides Jectofer active pharmaceutical ingredient (API) or Jectofer finished formulations upon request. The Jectofer suppliers may include Jectofer API manufacturers, exporters, distributors and traders.
click here to find a list of Jectofer suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Jectofer Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Jectofer GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Jectofer GMP manufacturer or Jectofer GMP API supplier for your needs.
A Jectofer CoA (Certificate of Analysis) is a formal document that attests to Jectofer's compliance with Jectofer specifications and serves as a tool for batch-level quality control.
Jectofer CoA mostly includes findings from lab analyses of a specific batch. For each Jectofer CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Jectofer may be tested according to a variety of international standards, such as European Pharmacopoeia (Jectofer EP), Jectofer JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Jectofer USP).
