
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
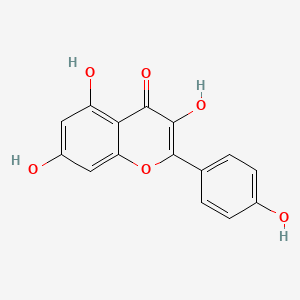

1. 3,4',5,7-tetrahydroxyflavone
2. Kempferol
1. 520-18-3
2. Robigenin
3. Kaempherol
4. Kempferol
5. Populnetin
6. Rhamnolutein
7. Trifolitin
8. Swartziol
9. 3,4',5,7-tetrahydroxyflavone
10. Pelargidenolon
11. Rhamnolutin
12. Indigo Yellow
13. Kampherol
14. Campherol
15. Kampferol
16. Nimbecetin
17. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4h-chromen-4-one
18. Kaemferol
19. 5,7,4'-trihydroxyflavonol
20. Pelargidenolon 1497
21. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4h-1-benzopyran-4-one
22. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one
23. C.i. 75640
24. 4h-1-benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-
25. Ccris 41
26. Flavone, 3,4',5,7-tetrahydroxy-
27. Pelargidenon
28. Kampcetin
29. Kempferol;robigenin
30. Nsc 407289
31. Nsc 656277
32. Mfcd00016938
33. Chembl150
34. Nsc-407289
35. Nsc-656277
36. Chebi:28499
37. 731p2le49e
38. Nsc656277
39. Cas-520-18-3
40. Dsstox_cid_768
41. Dsstox_rid_75781
42. Dsstox_gsid_20768
43. Smr000112585
44. Einecs 208-287-6
45. Brn 0304401
46. Unii-731p2le49e
47. Ai3-36096
48. 3,5,7,4'-tetrahydroxyflavone
49. Hsdb 7703
50. 4det
51. Kaempferol,(s)
52. Kaempferol [mi]
53. 5,4'-trihydroxyflavonol
54. Prestwick0_001098
55. Prestwick1_001098
56. Prestwick2_001098
57. Prestwick3_001098
58. 3'-deoxyquercetin
59. Kaempferol [hsdb]
60. Kaempferol [iarc]
61. Kaempferol [inci]
62. 3,5,7-tetrahydroxyflavone
63. 4',5,7-trihydroxyflavonol
64. Kaempferol [usp-rs]
65. Bidd:pxr0073
66. Oprea1_650954
67. Schembl18817
68. Bspbio_001176
69. 5-18-05-00251 (beilstein Handbook Reference)
70. Mls000697730
71. Mls001055391
72. Mls001074884
73. Mls006010737
74. Bidd:er0134
75. Spbio_003058
76. Kaempferol, Analytical Standard
77. Bdbm7462
78. Bpbio1_001294
79. Megxp0_001283
80. Dtxsid7020768
81. Flavone,4',5,7-tetrahydroxy-
82. Acon1_001867
83. Cid_5280863
84. Gtpl11052
85. Chebi: 28499
86. Hms1571k18
87. Hms2098k18
88. Hms2267i09
89. Hms3414c03
90. Hms3656m03
91. Hms3678c03
92. Hms3884b13
93. Kaempferol, >=97.0% (hplc)
94. Tnp00039
95. Zinc3869768
96. Tox21_201165
97. Tox21_303363
98. Ac-544
99. Hsci1_000027
100. Lmpk12110003
101. Nsc407289
102. S2314
103. Akos015895240
104. Kaempferol, >=90% (hplc), Powder
105. Ccg-202823
106. Cs-1273
107. Db01852
108. Gs-3570
109. Ncgc00016480-01
110. Ncgc00016480-02
111. Ncgc00016480-03
112. Ncgc00016480-04
113. Ncgc00016480-05
114. Ncgc00016480-06
115. Ncgc00016480-07
116. Ncgc00016480-08
117. Ncgc00016480-09
118. Ncgc00091036-01
119. Ncgc00091036-02
120. Ncgc00164322-01
121. Ncgc00179275-01
122. Ncgc00179275-02
123. Ncgc00257464-01
124. Ncgc00258717-01
125. Bp-25390
126. Ci 75640
127. Hy-14590
128. Kaempferol 100 Microg/ml In Acetonitrile
129. Sy023424
130. Ab00514046
131. Ft-0614420
132. K0018
133. N1719
134. Sw197199-2
135. 3,4',5,7-tetrahydroxy-flavone (7ci,8ci)
136. C05903
137. H10428
138. S00111
139. Flavone, 3,4',5,7-tetrahydroxy- (7ci,8ci)
140. Kaempferol (constituent Of Ginkgo) [dsc]
141. 520k183
142. A828886
143. Q393336
144. Sr-01000765646
145. Kaempferol, Primary Pharmaceutical Reference Standard
146. Q-100584
147. Sr-01000765646-3
148. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-chromen-4-one
149. Brd-k12807006-001-05-2
150. Brd-k12807006-001-10-2
151. 2-(4-hydroxyphenyl)-3,5,7-tris(oxidanyl)chromen-4-one
152. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one??
153. A91a6666-86c8-4b33-b3ef-f74cd3cd7f47
154. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1-benzopyran-4-one
155. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4h-chromen-4-one #
156. 4h-1-benzopyran-4-one,5,7-trihydroxy-2-(4-hydroxyphenyl)-
157. Kaempferol, United States Pharmacopeia (usp) Reference Standard
158. 4h-1-benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)- (9ci)
159. 3,4',5,7-tetrahydroxyflavone, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4h-1-benzopyran-4-one
| Molecular Weight | 286.24 g/mol |
|---|---|
| Molecular Formula | C15H10O6 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 286.04773803 g/mol |
| Monoisotopic Mass | 286.04773803 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 451 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL/ Despite recent advances in understanding molecular mechanisms involved in glioblastoma progression, the prognosis of the most malignant brain tumor continues to be dismal. Because the flavonoid kaempferol is known to suppress growth of a number of human malignancies, we investigated the effect of kaempferol on human glioblastoma cells. Kaempferol induced apoptosis in glioma cells by elevating intracellular oxidative stress. Heightened oxidative stress was characterized by an increased generation of reactive oxygen species (ROS) accompanied by a decrease in oxidant-scavenging agents such as superoxide dismutase (SOD-1) and thioredoxin (TRX-1). Knockdown of SOD-1 and TRX-1 expression by small interfering RNA (siRNA) increased ROS generation and sensitivity of glioma cells to kaempferol-induced apoptosis. Signs of apoptosis included decreased expression of Bcl-2 and altered mitochondrial membrane potential with elevated active caspase-3 and cleaved poly(ADP-ribose) polymerase expression. Plasma membrane potential and membrane fluidity were altered in kaempferol-treated cells. Kaempferol suppressed the expression of proinflammatory cytokine interleukin-6 and chemokines interleukin-8, monocyte chemoattractant protein-1, and regulated on activation, normal T-cell expressed and secreted. Kaempferol inhibited glioma cell migration in a ROS-dependent manner. Importantly, kaempferol potentiated the toxic effect of chemotherapeutic agent doxorubicin by amplifying ROS toxicity and decreasing the efflux of doxorubicin. Because the toxic effect of both kaempferol and doxorubicin was amplified when used in combination, this study raises the possibility of combinatorial therapy whose basis constitutes enhancing redox perturbation as a strategy to kill glioma cells.
PMID:17876051 Sharma V et al; Mol Cancer Ther 6 (9): 2544-53 (2007)
/EXPL/ Kaempferol is one of the most important constituents in ginkgo flavonoids. Recent studies indicate kaempferol may have antitumor activities. The objective of this study was to determine the effect and mechanisms of kaempferol on pancreatic cancer cell proliferation and apoptosis. Pancreatic cancer cell lines MIA PaCa-2 and Panc-1 were treated with kaempferol, and the inhibitory effects of kaempferol on pancreatic cancer cell proliferation were examined by direct cell counting, 3H-thymidine incorporation, and MTS assay. Lactate dehydrogenase release from cells was determined as an index of cytotoxicity. Apoptosis was analyzed by terminal deoxynucleotidyl transferase mediated dUTP nick end labeling assay. Upon the treatment with 70 microm kaempferol for 4 days, MIA PaCa-2 cell proliferation was significantly inhibited by 79% and 45.7% as determined by direct cell counting and MTS assay, respectively, compared with control cells (P < 0.05). Similarly, the treatment with kaempferol significantly inhibited Panc-1 cell proliferation. Kaempferol treatment also significantly reduced 3H-thymidine incorporation in both MIA PaCa-2 and Panc-1 cells. Combination treatment of low concentrations of kaempferol and 5-fluorouracil showed an additive effect on the inhibition of MIA PaCa-2 cell proliferation. Furthermore, kaempferol had significantly less cytotoxicity than 5-fluorouracil in normal human pancreatic ductal epithelial cells (P = 0.029). In both MIA PaCa-2 and Panc-1 cells, apoptotic cell population was increased when treated with kaempferol in a concentration-dependent manner. CONCLUSIONS: Ginkgo biloba extract kaempferol effectively inhibits pancreatic cancer cell proliferation and induces cancer cell apoptosis, which may sensitize pancreatic tumor cells to chemotherapy. Kaempferol may have clinical applications as adjuvant therapy in the treatment of pancreatic cancer.
PMID:18570926 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2924764 Zhang Y et al; J Surg Res 148 (1): 17-23 (2008)
/EXPL/ Dietary flavonols have been found to possess preventive and therapeutic potential against several kinds of cancers. This study is conducted to investigate the anti-proliferation effects of kaempferol, a major component of food flavonols, against colon cancer cells. In the human HCT116 colon cancer cell line, kaempferol induced p53-dependent growth inhibition and apoptosis. Furthermore, kaempferol was found to induce cytochrome c release from mitochondria and activate caspase-3 cleavage. The Bcl-2 family proteins including PUMA were involved in this process. Kaempferol also induced ATM and H2AX phosphorylation in HCT116 cells, inhibition of ATM by a chemical inhibitor resulted in abrogation of the downstream apoptotic cascades. These findings suggest kaempferol could be a potent candidate for colorectal cancer management.
PMID:19028473 Li W et al; Chem Biol Interact 177 (2): 121-7 (2009)
/EXPL/ ... Treatment of the chronic myelogenous leukemia cell line K562 and promyelocitic human leukemia U937 with 50 microM kaempferol resulted in an increase of the antioxidant enzymes Mn and Cu/Zn superoxide dismutase (SOD). Kaempferol treatment induced apoptosis by decreasing the expression of Bcl-2 and increasing the expressions of Bax. There were also induction of mitochondrial release of cytochrome c into cytosol and significant activation of caspase-3, and -9 with PARP cleavage. Kaempferol treatment increased the expression and the mitochondria localization of the NAD-dependent deacetylase SIRT3. K562 cells stably overexpressing SIRT3 were more sensitive to kaempferol, whereas SIRT3 silencing did not increase the resistance of K562 cells to kaempferol. Inhibition of PI3K and de-phosphorylation of Akt at Ser473 and Thr308 was also observed after treating both K562 and U937 cells with kaempferol. ... Oxidative stress induced by kaempferol in K562 and U937 cell lines causes the inactivation of Akt and the activation of the mitochondrial phase of the apoptotic program with an increase of Bax and SIRT3, decrease of Bcl-2, release of cytochrome c, caspase-3 activation, and cell death.
PMID:19160423 Marfe G et al; J Cell Biochem 106 (4): 643-50 (2009)
/EXPL/ Atherosclerosis is a chronic inflammatory disease of the arterial wall. Kaempferol and rhamnocitrin (kaempferol 7-O-methyl ether) are two anti-inflammatory flavonoids commonly found in plants. The aim of this study is to investigate the function of kaempferol and rhamnocitrin on prevention of atherosclerosis. Chemical analyses demonstrated that kaempferol and rhamnocitrin were scavengers of DPPH (1,1-diphenyl-2-picrylhydrazyl) with IC50 of 26.10 +/- 1.33 and 28.38 +/- 3.07 microM, respectively. Copper-induced low-density lipoprotein (LDL) oxidation was inhibited by kaempferol and rhamnocitrin, with similar potency, as measured by decreased formation of malondialdehyde and relative electrophoretic mobility (REM) on agarose gel, while rhamnocitrin reduced delayed formation of conjugated dienes better than kaempferol. Cholesterol-laden macrophages are the hallmark of atherogenesis. The class B scavenger receptor, CD36, binds oxidized low-density lipoprotein (oxLDL), is found in atherosclerotic lesions, and is up-regulated by oxLDL. Addition of kaempferol and rhamnocitrin (20 microM) caused significant reductions in cell surface CD36 protein expression in THP-1-derived macrophages (p < 0.05). Reverse transcription quantitative PCR (RT-Q-PCR) showed that kaempferol and rhamnocitrin (20 microM) decreased oxLDL-induced CD36 mRNA expression (p < 0.01 and p < 0.05, respectively). Kaempferol- and rhamnocitrin-treated macrophages also showed reduction in 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanide perchlorate (DiI)-labeled oxLDL uptake. Current evidences indicate that kaempferol and rhamnocitrin not only protect LDL from oxidation but also prevent atherogenesis through suppressing macrophage uptake of oxLDL.
PMID:17973448 Tu YC ET AL; J Agric Food Chem 55 (24): 9969-76 (2007)
The aim of this study was to assess kaempferol bioavailability in healthy humans, after bean (Phaseolus vulgaris L.) consumption through the monitoring of the excretion in relation to intake. In seven healthy subjects receiving kaempferol from cooked bean, maximum excretion of hydrolyzed flavonol was obtained after 2-8 hr. Intersexual variations in urinary excretion were found to be 6.10+/-5.50% and 5.40+/-5.40% of the kaempferol dose for male and female subjects, respectively. Although a 6.72-fold inter-individual variation between the highest and lowest excretion concentrations was found, all individuals exhibited similar excretion profiles. Moreover, a direct correlation between the percentage of kaempferol excreted and the body mass index of volunteers was observed with a correlation index equal to 0.80. All except two individuals exhibited a first peak of kaempferol excretion 2 hr after ingestion. The study reveals information about inter-individual excretion capacity after kaempferol intake and that kaempferol can be used as a biomarker for flavonol consumption.
PMID:17566888 Bonetti A et al; Int J Food Sci Nutr 58 (4): 261-9 (2007)
... A pharmacokinetic study of kaempferol from endive ... /was studied in / four healthy males and four healthy females. Kaempferol, from a relatively low dose (9 mg), was absorbed from endive with a mean maximum plasma concentration of 0.1 uM, at a time of 5.8 hr, indicating absorption from the distal section of the small intestine and/or the colon. Although a 7.5-fold interindividual variation between the highest and lowest maximum plasma concentration was observed, most individuals showed remarkably consistent pharmacokinetic profiles. This contrasts with profiles for other flavonoids that are absorbed predominantly from the large intestine (eg rutin). An average of 1.9% of the kaempferol dose was excreted in 24 hr. Most subjects also showed an early absorption peak, probably corresponding to kaempferol-3-glucoside, present at a level of 14% in the endive. Kaempferol-3-glucuronide was the major compound detected in plasma and urine. Quercetin was not detected in plasma or urine indicating a lack of phase I hydroxylation of kaempferol. Kaempferol is absorbed more efficiently than quercetin in humans even at low oral doses. The predominant form in plasma is a 3-glucuronide conjugate, and interindividual variation in absorption and excretion is low, suggesting that urinary kaempferol could be used as a biomarker for exposure.
PMID:15164116 DuPont MS et al; Eur J Clin Nutr 58 (6): 947-54 (2004)
Ten adult volunteers with an average age 28 years were given a single oral dose of six tablets of Ginkgo biloba extract. Quercetin and kaempferol in different period of human urine were determined by using RP-HPLC. The results showed the elimination rate constant k and the absorption rate constant ka of quercetin were slightly more than that of kaempferol; and the absorption half-life (t(1/2a)), the elimination half-life (t(1/2)) and t(max) of quercetin were less than that of kaempferol, the differences were, however, not statistically significant. The mean values of ka were 0.61 hr(-1) and 0.55 hr(-1), t(1/2a) 1.51 hr and 1.56 hr, k 0.37 hr(-1) and 0.30 hr(-1), t(1/2) 2.17 hr and 2.76 hr, T(max) 2.30 hr and 2.68 hr for quercetin and kaempferol, respectively, which mean absorption and elimination of quercetin and kaempferol are 0.17% and 0.22%, respectively. Quercetin and kaempferol are excreted in the human urine mainly as glucuronides.
PMID:14527089 Wang FM et al; Eur J Drug Metab Pharmacokinet 28 (3): 173-7 (2003)
The objective of this study was to investigate whether kaempferol and quercetin could be transported into primary cultured cerebral neurons, to establish a practical HPLC method with UV detection for the two flavonols in the neurons, and to study the uptake and transport behaviors of them through the neurons. The present results showed that the level of kaempferol in the neurons increased linearly and then reached a plateau with incubation time at the high concentration of 10 ?g/mL, but not at the other two concentrations of 1 and 0.1 ug/mL. However, the levels of quercetin in the neurons were not detected at the three incubating concentrations, and there was a new peak detected in the cell whose retention time was shorter (3.42 min) than that of quercetin (4.65 min). This phenomenon suggested that quercetin might be transported into the neurons and then metabolized quickly to some derivative. Kaempferol could be transported into the neurons in a concentration- and time-dependent manner when the neurons were incubated with the culture medium containing kaempferol at the high dose. There was an apparent correlation between the concentrations of kaempferol in the medium and in the cell, indicating that the uptake of kaempferol in the cell increased along with its dose (10 ug/mL). However, there was a negative correlation between the concentrations of quercetin in the medium and in the cell. The results suggested that kaempferol and quercetin were disposed by the neurons at different way, and this might be an important factor for their different effects on primary cultured cortical cells.
PMID:16799931 Liu R et al; Biomed Chromatogr 20 (11): 1178-1184 (2006)
To elucidate the metabolism of hispidulin in the large intestine, its biotransformation by the pig caecal microflora was studied. In addition, the efficiency of the pig caecal microflora to degrade galangin (3,5,7-trihydroxyflavone), kaempferol (3,5,7,4?-tetrahydroxyflavone), apigenin (5,7,4?-trihydroxyflavone), and luteolin (5,7,3?,4?- tetrahydroxyflavone) was investigated. Identification of the formed metabolites was performed by high-performance liquid chromatography (HPLC)-diode array detection, HPLC-electrospray ionization-tandem mass spectrometry, and high-resolution gas chromatography-mass spectrometry. The caecal microflora transformed ... kaempferol to 4-hydroxyphenylacetic acid, phloroglucinol, and 4-methylphenol; ... To elucidate to what extent different hydroxylation patterns on the B-ring influence the degradation degree of flavonoids, the conversions of galangin and kaempferol as well as that of apigenin and luteolin were compared with those of quercetin (3,5,7,3?, 4?-pentahydroxyflavone) and chrysin (5.7-dihydroxyflavone), respectively. Regardless of the flavonoid subclass, the presence of a hydroxy group at the 4?-position seems to be a prerequisite for fast breakdown. An additional hydroxy group at the B-ring did not affect the degradation degree.
PMID:16317785 Labib S et al; Mol Nutr Food Res 50 (1): 78-86 (2006)
The metabolism of the flavonoids quercetin and kaempferol by rat hepatocytes was investigated using liquid chromatography coupled with electrospray mass spectrometry (LC-ESI MS). Quercetin and kaempferol were extensively metabolized (98.8 +/- 0.1% and 81.0 +/- 5.1% respectively, n = 4), with four glucuronides of quercetin and two of kaempferol being detected after incubation. The glucuronides of quercetin and kaempferol formed upon incubation with rat hepatocytes were identified as the same ones formed after incubation with the UDP-glucuronosyltransferase isoform UGT1A9. In addition, plasma samples from human volunteers taken after consumption of capsules of Ginkgo biloba, a plant rich in flavonoid glycosides, were analysed by LC-MS for the presence of flavonoid glucuronides and flavonoid glycosides. Reported is evidence for the presence of flavonoid glycosides in samples of plasma. The results suggest that UGT1A9 is a key UDP-glucuronosyltransferase isoform for the metabolism of flavonoids, and that absorption of intact flavonoid glycosides is possible.
PMID:12028662 Oliveira EJ et al; Xenobiotica 32 (4): 279-87 (200)
Kaempferol is a flavonoid widely distributed in edible plants and has been shown to be genotoxic to V79 cells in the absence of external metabolizing systems. The presence of an external metabolizing system, such as rat liver homogenates (S9 mix), leads to an increase in its genotoxicity, which is attributed to its biotransformation to the more genotoxic flavonoid quercetin, via the cytochrome P450 (CYP) mono-oxygenase system. ...
PMID:9379919 Silva ID et al; Mutagenesis 12 (5): 383-90 (1997)
Kaempferol has known human metabolites that include (2S,3S,4S,5R)-6-[3,5-Dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid and Kaempferol-3-glucuronide.
Kaempferol is a known human metabolite of galangin and kaempferide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Ten adult volunteers with an average age 28 years were given a single oral dose of six tablets of Ginkgo biloba extract. ... The absorption half life was 1.51 hr and elimination half-life was 1.56 hr.
PMID:14527089 Wang FM et al; Eur J Drug Metab Pharmacokinet 28 (3): 173-7 (2003)
Pure kaempferol and a number of related flavonoids were examined as MAOIs in-vitro. Kaempferol, apigenin and chrysin proved to be potent monoamine oxidase (MAO) inhibitors (MAOI)s, but produced more pronounced inhibition of MAO-A than MAO-B. IC50 (50% inhibition concentration) values for the ability of these three flavones to inhibit MAO-A were 7 x 10(-7), 1 x 10(-6) and 2 x 10(-6) M, respectively. Ginkgo biloba leaf extract and kaempferol were found to have no effect ex-vivo on rat or mouse brain MAO or on concentrations of dopamine, noradrenaline, 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Kaempferol was shown to protect against N-methyl-D-aspartate-induced neuronal toxicity in-vitro in rat cortical cultures, but did not prevent DSP-4-induced noradrenergic neurotoxicity in an in-vivo model. Both Ginkgo biloba extract and kaempferol were demonstrated to be antioxidants in a lipid-peroxidation assay. This data indicates that the MAO-inhibiting activity of Ginkgo biloba extract is primarily due to the presence of kaempferol. Ginkgo biloba extract has properties indicative of potential neuroprotective ability.
PMID:10813558 Sloley BD et al; J Pharm Pharmacol 52 (4): 451-9 (2000)
Kaempferol is a dietary flavonoid that is thought to function as a selective estrogen receptor modulator. ... This study ... established that kaempferol also functions as an inverse agonist for estrogen-related receptors alpha and gamma (ERRalpha and ERRgamma). ... Kaempferol binds to ERRalpha and ERRgamma and blocks their interaction with coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha). Kaempferol also suppressed the expressions of ERR-target genes pyruvate dehydrogenase kinase 2 and 4 (PDK2 and PDK4). This evidence suggests that kaempferol may exert some of its biological effect through both estrogen receptors and estrogen-related receptors.
PMID:19171140 Wang J et al; FEBS Lett 583 (4): 643-7 (2009)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
55
PharmaCompass offers a list of Kaempferol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Kaempferol manufacturer or Kaempferol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Kaempferol manufacturer or Kaempferol supplier.
PharmaCompass also assists you with knowing the Kaempferol API Price utilized in the formulation of products. Kaempferol API Price is not always fixed or binding as the Kaempferol Price is obtained through a variety of data sources. The Kaempferol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Kaempferol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Kaempferol, including repackagers and relabelers. The FDA regulates Kaempferol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Kaempferol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Kaempferol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Kaempferol supplier is an individual or a company that provides Kaempferol active pharmaceutical ingredient (API) or Kaempferol finished formulations upon request. The Kaempferol suppliers may include Kaempferol API manufacturers, exporters, distributors and traders.
click here to find a list of Kaempferol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Kaempferol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Kaempferol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Kaempferol GMP manufacturer or Kaempferol GMP API supplier for your needs.
A Kaempferol CoA (Certificate of Analysis) is a formal document that attests to Kaempferol's compliance with Kaempferol specifications and serves as a tool for batch-level quality control.
Kaempferol CoA mostly includes findings from lab analyses of a specific batch. For each Kaempferol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Kaempferol may be tested according to a variety of international standards, such as European Pharmacopoeia (Kaempferol EP), Kaempferol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Kaempferol USP).

