
Synopsis
Synopsis
0
JDMF
0
VMF
0
Australia

Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
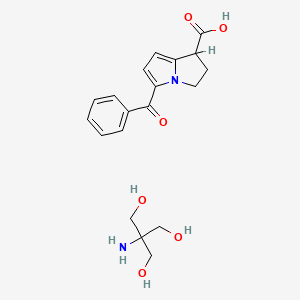

1. Acular
2. Toradol
1. 74103-07-4
2. Ketorolac Tris Salt
3. Toradol
4. Acular
5. Ketorolac (tromethamine Salt)
6. Ketorolac Tromethamine Salt
7. Ketorolac Trometamol
8. Acular Ls
9. Acuvail
10. Lixidol
11. Acular Pf
12. Sprix
13. Dolac
14. Tarasyn
15. Ketorolac (toradol)
16. 2-amino-2-(hydroxymethyl)propane-1,3-diol 5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylate
17. Nsc-758637
18. 4eve5946bq
19. Syntex
20. Toradol (tn)
21. (+/-)-5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylic Acid Tris Salt
22. 2-amino-2-(hydroxymethyl)propane-1,3-diol;5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylic Acid
23. Godek
24. Acular Preservative Free
25. Ketorolac Tromethamine;ketorolac Tris Salt;rs37619 Tromethamine Salt
26. Chebi:6130
27. Smr000058461
28. Ketorolac Tromethamine [usan]
29. Tromethamine Ketorolac
30. Sr-01000075948
31. Rs-37619
32. Unii-4eve5946bq
33. Toratex
34. Droal
35. Ketorolactrissalt
36. Tora-dol
37. Ketorolac Tromethamine [usan:usp]
38. Acular (tn)
39. Mfcd00887595
40. Bppc
41. Spectrum_001578
42. Cpd000058461
43. Ketorolac Tromethamine Ophthalmic Solution
44. Prestwick0_000929
45. Prestwick1_000929
46. Prestwick2_000929
47. Prestwick3_000929
48. Spectrum2_001598
49. Spectrum3_001975
50. Spectrum4_000215
51. Spectrum5_001273
52. Ketrolac.tromethamine Salt
53. Schembl5036
54. Lopac0_000676
55. Bspbio_000838
56. Bspbio_003575
57. Kbiogr_000849
58. Kbioss_002058
59. (+-)-5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylic Acid, Compound With 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
60. 2-amino-2-(hydroxymethyl)propane-1,3-diol; 5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylic Acid
61. Ketorolac Tromethamine (usp)
62. Mls000069689
63. Mls001401455
64. Mls002222310
65. Divk1c_000836
66. Spectrum1503925
67. Spbio_001596
68. Spbio_003017
69. Bpbio1_000922
70. Chembl1201124
71. Dtxsid0045597
72. Hms502j18
73. Kbio1_000836
74. Kbio2_002058
75. Kbio2_004626
76. Kbio2_007194
77. Kbio3_002953
78. Rox-828
79. Rox-888
80. Ninds_000836
81. Hms1570j20
82. Hms1922k22
83. Hms2051p06
84. Hms2093m05
85. Hms2097j20
86. Hms2235n13
87. Hms3262g13
88. Hms3373m06
89. Hms3393p06
90. Hms3655e15
91. Hms3714j20
92. Ketorolac Trometamol [jan]
93. Pharmakon1600-01503925
94. Bcp02917
95. Hy-b0138
96. (+/-)-ketorolac Tromethamine Salt
97. Tox21_500676
98. Ccg-39376
99. Ketorolac Trometamol [mart.]
100. Nsc758637
101. S5698
102. Ketorolac Tromethamine [vandf]
103. Akos024386301
104. Bcp9000811
105. Ccg-101006
106. Cs-1933
107. Ketorolac Tromethamine [usp-rs]
108. Ketorolac Tromethamine [who-dd]
109. Lp00676
110. Nc00256
111. Nsc 758637
112. Idi1_000836
113. Ncgc00016159-02
114. Ncgc00016159-03
115. Ncgc00016159-04
116. Ncgc00094036-01
117. Ncgc00094036-02
118. Ncgc00094036-03
119. Ncgc00261361-01
120. 1h-pyrrolizine-1-carboxylic Acid, 5-benzoyl-2,3-dihydro, (+-)-, Compound With 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
121. As-15193
122. Ketorolac Tris Salt;ketorolac Tromethamine
123. Bcp0726000301
124. Ketorolac Tris Salt, >=99%, Crystalline
125. Ketorolac Trometamol [ep Monograph]
126. Db-055837
127. Ketorolac Trometamol For Peak Identification
128. Ketorolac Tromethamine [orange Book]
129. Eu-0100676
130. Ft-0630829
131. K0053
132. Sw197293-4
133. Ketorolac Tromethamine [usp Monograph]
134. D00813
135. K 1136
136. M02042
137. Omidria Component Ketorolac Tromethamine
138. 103k074
139. A838011
140. Ketorolac Tromethamine Component Of Omidria
141. Sr-01000946595
142. Q-201269
143. Sr-01000075948-1
144. Sr-01000075948-6
145. Sr-01000075948-8
146. Sr-01000946595-1
147. Ketorolac (+/-)-form Tromethamine Salt [mi]
148. Q27107089
149. F0001-2390
150. Ketorolac Trometamol 1.0 Mg/ml In Methanol (as Free Acid)
151. Ketorolac Trometamol, European Pharmacopoeia (ep) Reference Standard
152. Ketorolac Tromethamine, United States Pharmacopeia (usp) Reference Standard
153. Ketorolac Trometamol For Peak Identification, European Pharmacopoeia (ep) Reference Standard
154. Ketorolac Tromethamine, Pharmaceutical Secondary Standard; Certified Reference Material
155. (+/-)-5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylic Acid, Compound With 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
156. 1h-pyrrolizine-1-1h-pyrrolizine-1-carboxylic Acid, 5-benzoyl-2,3-dihydro, (+/-)-, Compound With 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
157. 2-amino-2-(hydroxymethyl)propane-1,3-diol (1rs)-5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylate
158. 5-benzoyl-2,3-dihydro-1h-pyrrolizine-1-carboxylate; 2-hydroxyethyl-bis(hydroxymethyl)ammonium;ketorolac Tromethamine
1. Trometamol Ketorolac
| Molecular Weight | 376.4 g/mol |
|---|---|
| Molecular Formula | C19H24N2O6 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 376.16343649 g/mol |
| Monoisotopic Mass | 376.16343649 g/mol |
| Topological Polar Surface Area | 146 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 430 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 10 | |
|---|---|
| Drug Name | Acular |
| PubMed Health | Ketorolac |
| Drug Classes | Analgesic, Central Nervous System Agent, Ophthalmologic Agent |
| Drug Label | ACULAR (ketorolac tromethamine ophthalmic solution) is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is ()-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, comp... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 10 | |
|---|---|
| Drug Name | Acular ls |
| PubMed Health | Ketorolac (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | ACULAR LS (ketorolac tromethamine ophthalmic solution) 0.4% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Structural and Molecular Formula: C19H24N2O6Mol Wt 376.40Chemical Name... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.4% |
| Market Status | Prescription |
| Company | Allergan |
| 3 of 10 | |
|---|---|
| Drug Name | Acuvail |
| PubMed Health | Ketorolac |
| Drug Classes | Analgesic, Central Nervous System Agent, Ophthalmologic Agent |
| Drug Label | ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is ()-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic aci... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.45% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 10 | |
|---|---|
| Drug Name | Ketorolac tromethamine |
| Drug Label | Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of non-steroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine is (acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol, and the structural form... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops; Tablet; Injectable |
| Route | injection; Ophthalmic; Injection; Oral |
| Strength | 0.5%; 0.4%; 30mg/ml; 60mg/2ml; 10mg; 15mg/ml |
| Market Status | Prescription |
| Company | Wockhardt; Hospira; Gland Pharma; Teva; Apotex; Sandoz; Sun Pharma Global; Fresenius Kabi Usa; Hikma Maple; Alcon Pharms; Amphastar Pharm; Sagent Pharms; Pliva; Agila Speclts; Mylan; Akorn |
| 5 of 10 | |
|---|---|
| Drug Name | Sprix |
| Drug Label | Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine is ()-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hy... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 15.75mg/spray |
| Market Status | Prescription |
| Company | Luitpold |
| 6 of 10 | |
|---|---|
| Drug Name | Acular |
| PubMed Health | Ketorolac |
| Drug Classes | Analgesic, Central Nervous System Agent, Ophthalmologic Agent |
| Drug Label | ACULAR (ketorolac tromethamine ophthalmic solution) is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is ()-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, comp... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Allergan |
| 7 of 10 | |
|---|---|
| Drug Name | Acular ls |
| PubMed Health | Ketorolac (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | ACULAR LS (ketorolac tromethamine ophthalmic solution) 0.4% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Structural and Molecular Formula: C19H24N2O6Mol Wt 376.40Chemical Name... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.4% |
| Market Status | Prescription |
| Company | Allergan |
| 8 of 10 | |
|---|---|
| Drug Name | Acuvail |
| PubMed Health | Ketorolac |
| Drug Classes | Analgesic, Central Nervous System Agent, Ophthalmologic Agent |
| Drug Label | ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is ()-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic aci... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.45% |
| Market Status | Prescription |
| Company | Allergan |
| 9 of 10 | |
|---|---|
| Drug Name | Ketorolac tromethamine |
| Drug Label | Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of non-steroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine is (acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol, and the structural form... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Solution/drops; Tablet; Injectable |
| Route | injection; Ophthalmic; Injection; Oral |
| Strength | 0.5%; 0.4%; 30mg/ml; 60mg/2ml; 10mg; 15mg/ml |
| Market Status | Prescription |
| Company | Wockhardt; Hospira; Gland Pharma; Teva; Apotex; Sandoz; Sun Pharma Global; Fresenius Kabi Usa; Hikma Maple; Alcon Pharms; Amphastar Pharm; Sagent Pharms; Pliva; Agila Speclts; Mylan; Akorn |
| 10 of 10 | |
|---|---|
| Drug Name | Sprix |
| Drug Label | Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine is ()-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hy... |
| Active Ingredient | Ketorolac tromethamine |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 15.75mg/spray |
| Market Status | Prescription |
| Company | Luitpold |
Investigated for use/treatment in pain (acute or chronic).
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
ROX-888 is a intranasal formulation of the broadly used injectible analgesic, ketorolac. It has ability to provide effective analgesia in acute medical conditions resulting in moderate-severe pain, without the disabling side effects of opioid analgesics
About the Company : Minakem Montreal is developing and manufacturing small molecules APIs and advanced intermediates, including corticosteroids. Following efficient processes and methodologies, our em...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
About the Company : Gonane Pharma, is a contract pharmaceutical company located in Gujarat, India, specializing in the manufacturing and marketing of Corticosteroids, Hormones, Antivirals, and Oncolog...
About the Company : Jai Radhe Sales was founded in 1999 as an out-of-the-box distribution firm specializing in the global supply of high-quality pharmaceutical ingredients. The firm provides complete ...
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
About the Company : Faran Shimi Pharmaceutical Company, established in 2001 and affiliated with Golrang Pharmaceutical Investment Co, manufactures high-quality Active Pharmaceutical Ingredients (APIs)...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
About the Company : Atman Pharma Private Limited is a fully integrated pharmaceutical company that has distinguished itself as a leader in Bulk Drugs (API) marketing both domestically in India and ove...
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
About the Company : Zeal MediPharma is a globally recognized Star One Export House, serving customers in over 50 countries for more than two decades. We specialize in sourcing and exporting high-quali...
About the Company : Alembic Pharmaceuticals Limited is a leading pharmaceutical company in India. The Company is vertically integrated with the ability to develop, manufacture and market pharmaceutica...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
75
PharmaCompass offers a list of Ketorolac Trometamol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ketorolac Trometamol manufacturer or Ketorolac Trometamol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ketorolac Trometamol manufacturer or Ketorolac Trometamol supplier.
PharmaCompass also assists you with knowing the Ketorolac Trometamol API Price utilized in the formulation of products. Ketorolac Trometamol API Price is not always fixed or binding as the Ketorolac Trometamol Price is obtained through a variety of data sources. The Ketorolac Trometamol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ketorolac Tromethamine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ketorolac Tromethamine, including repackagers and relabelers. The FDA regulates Ketorolac Tromethamine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ketorolac Tromethamine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ketorolac Tromethamine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ketorolac Tromethamine supplier is an individual or a company that provides Ketorolac Tromethamine active pharmaceutical ingredient (API) or Ketorolac Tromethamine finished formulations upon request. The Ketorolac Tromethamine suppliers may include Ketorolac Tromethamine API manufacturers, exporters, distributors and traders.
click here to find a list of Ketorolac Tromethamine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ketorolac Tromethamine DMF (Drug Master File) is a document detailing the whole manufacturing process of Ketorolac Tromethamine active pharmaceutical ingredient (API) in detail. Different forms of Ketorolac Tromethamine DMFs exist exist since differing nations have different regulations, such as Ketorolac Tromethamine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ketorolac Tromethamine DMF submitted to regulatory agencies in the US is known as a USDMF. Ketorolac Tromethamine USDMF includes data on Ketorolac Tromethamine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ketorolac Tromethamine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ketorolac Tromethamine suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Ketorolac Tromethamine Drug Master File in Korea (Ketorolac Tromethamine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Ketorolac Tromethamine. The MFDS reviews the Ketorolac Tromethamine KDMF as part of the drug registration process and uses the information provided in the Ketorolac Tromethamine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Ketorolac Tromethamine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Ketorolac Tromethamine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Ketorolac Tromethamine suppliers with KDMF on PharmaCompass.
A Ketorolac Tromethamine CEP of the European Pharmacopoeia monograph is often referred to as a Ketorolac Tromethamine Certificate of Suitability (COS). The purpose of a Ketorolac Tromethamine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ketorolac Tromethamine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ketorolac Tromethamine to their clients by showing that a Ketorolac Tromethamine CEP has been issued for it. The manufacturer submits a Ketorolac Tromethamine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ketorolac Tromethamine CEP holder for the record. Additionally, the data presented in the Ketorolac Tromethamine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ketorolac Tromethamine DMF.
A Ketorolac Tromethamine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ketorolac Tromethamine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ketorolac Tromethamine suppliers with CEP (COS) on PharmaCompass.
A Ketorolac Tromethamine written confirmation (Ketorolac Tromethamine WC) is an official document issued by a regulatory agency to a Ketorolac Tromethamine manufacturer, verifying that the manufacturing facility of a Ketorolac Tromethamine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ketorolac Tromethamine APIs or Ketorolac Tromethamine finished pharmaceutical products to another nation, regulatory agencies frequently require a Ketorolac Tromethamine WC (written confirmation) as part of the regulatory process.
click here to find a list of Ketorolac Tromethamine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ketorolac Tromethamine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ketorolac Tromethamine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ketorolac Tromethamine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ketorolac Tromethamine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ketorolac Tromethamine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ketorolac Tromethamine suppliers with NDC on PharmaCompass.
Ketorolac Tromethamine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ketorolac Tromethamine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ketorolac Tromethamine GMP manufacturer or Ketorolac Tromethamine GMP API supplier for your needs.
A Ketorolac Tromethamine CoA (Certificate of Analysis) is a formal document that attests to Ketorolac Tromethamine's compliance with Ketorolac Tromethamine specifications and serves as a tool for batch-level quality control.
Ketorolac Tromethamine CoA mostly includes findings from lab analyses of a specific batch. For each Ketorolac Tromethamine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ketorolac Tromethamine may be tested according to a variety of international standards, such as European Pharmacopoeia (Ketorolac Tromethamine EP), Ketorolac Tromethamine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ketorolac Tromethamine USP).
