
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
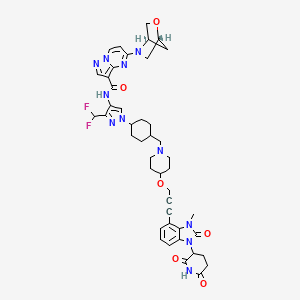

1. 2432994-31-3
2. Kt-474
3. Kt474
4. N-[3-(difluoromethyl)-1-[trans-4-[[4-[[3-[1-(2,6-dioxo-3-piperidinyl)-2,3-dihydro-3-methyl-2-oxo-1h-benzimidazol-4-yl]-2-propyn-1-yl]oxy]-1-piperidinyl]methyl]cyclohexyl]-1h-pyrazol-4-yl]-5-(1r,4r)-2-oxa-5-azabicyclo[2.2.1]hept-5-ylpyrazolo[1,5-a]pyrimidine-3-carboxamide
5. N-[3-(difluoromethyl)-1-[4-[[4-[3-[1-(2,6-dioxopiperidin-3-yl)-3-methyl-2-oxobenzimidazol-4-yl]prop-2-ynoxy]piperidin-1-yl]methyl]cyclohexyl]pyrazol-4-yl]-5-[(1r,4r)-2-oxa-5-azabicyclo[2.2.1]heptan-5-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide
6. 2sxr65p7e3
7. Schembl21998241
8. Schembl23807243
9. Schembl25986139
10. Gtpl12892
11. Kym-001
12. Glxc-26797
13. Glxc-27930
14. Ex-a8217
15. Kt-i-417
16. Sar444656
17. At41570
18. Kt-474?
19. Sar-444656
20. Compound A [wo2022174269a1]
21. Da-77150
22. Hy-145483
23. Cs-0374973
24. 5-((1r,4r)-2-oxa-5-azabicyclo[2.2.1]heptan-5-yl)-n-(3-(difluoromethyl)-1-((1r,4r)-4-((4-((3-(1-(2,6-dioxopiperidin-3-yl)-3-methyl-2-oxo-2,3-dihydro-1h-benzo[d]imidazol-4-yl)prop-2-yn-1-yl)oxy)piperidin-1-yl)methyl)cyclohexyl)-1h-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
25. Pyrazolo(1,5-a)pyrimidine-3-carboxamide, N-(3-(difluoromethyl)-1-(trans-4-((4-((3-(1-(2,6-dioxo-3-piperidinyl)-2,3-dihydro-3-methyl-2-oxo-1h-benzimidazol-4-yl)-2-propyn-1-yl)oxy)-1-piperidinyl)methyl)cyclohexyl)-1h-pyrazol-4-yl)-5-(1r,4r)-2-oxa-5-azabicyclo(2.2.1)hept-5-yl-
| Molecular Weight | 865.9 g/mol |
|---|---|
| Molecular Formula | C44H49F2N11O6 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 11 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 172 |
| Heavy Atom Count | 63 |
| Formal Charge | 0 |
| Complexity | 1790 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE
