
Synopsis
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Europe

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Cysteine Hydrochloride
2. Half Cystine
3. Half-cystine
4. L Cysteine
5. L-cysteine
6. Zinc Cysteinate
1. L-cysteine
2. 52-90-4
3. (r)-2-amino-3-mercaptopropanoic Acid
4. Thioserine
5. Cystein
6. Half-cystine
7. (r)-cysteine
8. L-(+)-cysteine
9. L-cystein
10. (2r)-2-amino-3-sulfanylpropanoic Acid
11. H-cys-oh
12. Cysteinum
13. Cysteine, L-
14. Beta-mercaptoalanine
15. Half Cystine
16. Free Cysteine
17. L-cys
18. L-alanine, 3-mercapto-
19. (r)-2-amino-3-mercaptopropionic Acid
20. Cisteinum [latin]
21. Cysteine [inn]
22. Fema No. 3263
23. L-2-amino-3-mercaptopropionic Acid
24. Cisteina [spanish]
25. Cisteina
26. Cisteinum
27. L Cysteine
28. L-zystein
29. Alpha-amino-beta-thiolpropionic Acid
30. L-2-amino-3-mercaptopropanoic Acid
31. Cysteinum [inn-latin]
32. Cisteina [inn-spanish]
33. Beta-mercaptoalanine, L-
34. (2r)-2-amino-3-mercaptopropanoic Acid
35. Nsc-8746
36. 2-amino-3-mercaptopropionic Acid
37. Ccris 912
38. Chebi:17561
39. Hsdb 2109
40. Alpha-amino-beta-thiolpropionic Acid, L-
41. Ai3-26559
42. Alpha-amino-beta-mercaptopropanoic Acid, L-
43. Alpha-amino-beta-mercaptopropionic Acid, L-
44. Propanoic Acid, 2-amino-3-mercapto-, (r)-
45. 2-amino-3-mercaptopropanoic Acid, (r)-
46. Cysteine Hcl
47. (r)-(+)-cysteine
48. E 920
49. Cys
50. Chembl863
51. E920
52. Mfcd00064306
53. K848jz4886
54. (+)-2-amino-3-mercaptopropionic Acid
55. 4371-52-2
56. Polycysteine
57. B-mercaptoalanine
58. Nsc 8746
59. (2r)-2-amino-3-mercaptopropanoate
60. (2r)-2-amino-3-sulfanylpropanoate
61. 3-mercapto-l-alanine
62. 202114-66-7
63. Einecs 200-158-2
64. L-cycteine
65. Racemic Cysteine
66. 2-amino-3-mercaptopropanoate
67. Unii-k848jz4886
68. 1ssq
69. Ecolan (tn)
70. L-cysteine, 97%
71. .beta.-mercaptoalanine
72. (2r)-2-amino-3-sulfanyl-propanoic Acid
73. 1xt8
74. Cysteine [hsdb]
75. Cysteine [inci]
76. L-cysteine (jp17)
77. Cysteine [ii]
78. Cysteine [mi]
79. Cysteine [vandf]
80. Cysteinum [hpus]
81. Cysteine [mart.]
82. L-cysteine [jan]
83. Bmse000034
84. Bmse000975
85. Cysteine [who-dd]
86. Epitope Id:140791
87. L-cysteine [fhfi]
88. L-cysteine-[1-13c]
89. Ec 200-158-2
90. L-cysteine [vandf]
91. 2-amino-3-mercaptopropionate
92. Gtpl4782
93. L-cysteine, >=97%, Fg
94. L-2-amino-3-mercaptopropanoate
95. 2-amino-3-mercapto-, (r)-
96. Dtxsid8022876
97. L-cysteine From Non-animal Source
98. Aids002953
99. Zinc895042
100. (r)-2-amino-3-mercaptopropanoate
101. Hy-y0337
102. Str02584
103. (r)-2-amino-3-mercapto-propanoate
104. Bdbm50109609
105. S5635
106. (r)-2-amino-3-mercaptopropanoicacid
107. Akos015854128
108. Am81648
109. Ccg-266077
110. Cs-w009027
111. Db00151
112. (r)-2-amino-3-mercapto-propanoic Acid
113. Ncgc00248803-01
114. .alpha.-amino-.beta.-thiolpropionic Acid
115. L-cysteine, Bioultra, >=98.5% (rt)
116. R-2-amino-3-mercaptopropionic Acid
117. E-920
118. L-cysteine, Saj Special Grade, >=98.5%
119. L-cysteine, Vetec(tm) Reagent Grade, 97%
120. Acetylcysteine Impurity B [ep Impurity]
121. C-9615
122. C00097
123. D00026
124. M03086
125. Propanoic Acid, 2-amino-3-mercapto-, (r)-
126. L-cysteine, Cell Culture Reagent (h-l-cys-oh)
127. 064c306
128. Q186474
129. (2r)-2-amino-3-sulfanylpropanoic Acid Hydrochloride
130. Q-201286
131. Q-201328
132. Bbae7ae6-fc21-4d37-808d-c7f7a2ba637f
133. Q27115093
134. F0001-2369
135. F8880-8973
136. L-cysteine, Certified Reference Material, Tracecert(r)
137. L-cysteine, Produced By Wacker Chemie Ag, Burghausen, Germany, >=98.0%
138. L-cysteine, From Non-animal Source, Bioreagent, Suitable For Cell Culture, >=98%
139. L-cysteine, Pharmagrade, From Non-animal Source, Ajinomoto, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
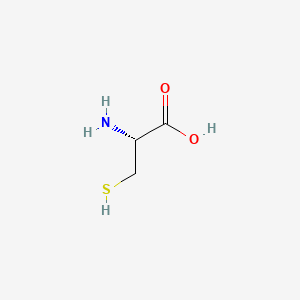
| Molecular Weight | 121.16 g/mol |
|---|---|
| Molecular Formula | C3H7NO2S |
| XLogP3 | -2.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 121.01974964 g/mol |
| Monoisotopic Mass | 121.01974964 g/mol |
| Topological Polar Surface Area | 64.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 75.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Acetaminophen-cysteine adducts (APAP-CYS) are a serum biomarker of acetaminophen exposure, formed when the oxidative metabolite of acetaminophen binds to cysteine residues of hepatic proteins. APAP-CYS adducts become elevated in cases of acute liver failure following acetaminophen overdose and have been proposed as a diagnostic tool to identify acetaminophen-induced acute liver failure when standard testing is inconclusive.
PMID:25896948 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4469719 Frey SM et al; J Med Toxicol 11 (2): 218-22 (2015)
/EXPL THER/ Lead is a toxic heavy metal that adversely affects nervous tissues; it often occurs as an environmental pollutant. We investigated histological changes in the cerebral cortex, hippocampus and cerebellum of adult albino mice following exposure to lead acetate. We also studied the possible ameliorative effect of the chelating agent, L-cysteine, on lead-induced neurotoxicity. We divided albino mice into six groups: 1) vehicle-only control, 2) L-cysteine control, 3 and 4) treated for 7 days with 20 and 40 mg/kg lead acetate, respectively, and 5 and 6) treated for 7 days with 20 and 40 mg/kg lead acetate, respectively, followed by 50 mg/kg L-cysteine for 7 days. Lead acetate administration caused disorganization of cell layers, neuronal loss and degeneration, and neuropil vacuolization. Brain sections from lead-intoxicated mice treated with L-cysteine showed fewer pathological changes; the neuropil showed less vacuolization and the neurons appeared less damaged. L-cysteine at the dose we used only marginally alleviated lead-induced toxicity.
PMID:27045382 Mahmoud YI, Sayed SS; Biotech Histochem 1-6 (2016) (Epub ahead of print)
/EXPL THER/ In hamster lung cell cultures addn of l-Cysteine or vit C to media protects against or reverses abnormal growth & malignant transformation in aged controls (1-2 yr old) or young (3-6 mo) after repeated exposure to smoke of tobacco or marijuana cigarettes.
Leuchtenberger C; Br J Exp Pathol 58 (6): 625-34 (1977)
/EXPL THER/ L-Cysteine admin orally or ip to rats protected against acute toxicity of methylmercury chloride, reducing mercury content in kidney & brain but not in liver.
Sugiyama et al; Toho Igakkai Zasshi 22 (1): 78-85 (1975)
For more Therapeutic Uses (Complete) data for CYSTEINE (7 total), please visit the HSDB record page.
For the prevention of liver damage and kidney damage associated with overdoses of acetaminophen
Due to this ability to undergo redox reactions, cysteine has antioxidant properties. Cysteine is an important source of sulfur in human metabolism, and although it is classified as a non-essential amino acid, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine may at some point be recognized as an essential or conditionally essential amino acid.
L-Cysteine is the central compound in sulfur metabolism in the human body. In proteins the formation of disulfide bonds between the thiol groups of cysteine plays an important role for tertiary structure and enzymatic activity; cysteine is however always incorporated in the polypeptide chain as cysteine. L-Cysteine is degraded to pyruvate in two steps: one is removal of sulfur and the other is a transamination. Cysteine can be metabolized to form taurine and carbon dioxide through the cysteinsulfinate pathway, where the initial step is oxidation of cysteine to cysteine sulfinate. This step is catalyzed by cysteine dioxygenase. Cysteine sulfinate may then be decarboxylated to form taurine or it may be metabolized via the putative intermediate beta-sulfinylpyruvate to pyruvate and sulfite and then to carbon dioxide and sulfate.
European Chemicals Agency (ECHA); Registered Substances, L-cysteine (CAS Number: 52-90-4) (EC Number: 200-158-2) (Last updated: May 17, 2016). Available from, as of May 24, 2016: https://echa.europa.eu/
Amino acid catabolism is essential for adjusting pool sizes of free amino acids and takes part in energy production as well as nutrient remobilization. The carbon skeletons are generally converted to precursors or intermediates of the tricarboxylic acid cycle. In the case of cysteine, the reduced sulfur derived from the thiol group also has to be oxidized in order to prevent accumulation to toxic concentrations. Here we present a mitochondrial sulfur catabolic pathway catalyzing the complete oxidation of L-cysteine to pyruvate and thiosulfate. After transamination to 3-mercaptopyruvate the sulfhydryl group from L-cysteine is transferred to glutathione by sulfurtransferase 1 and oxidized to sulfite by the sulfur dioxygenase ETHE1. Sulfite is then converted to thiosulfate by addition of a second persulfide group by sulfurtransferase 1. This pathway is most relevant during early embryo development and for vegetative growth under light limiting conditions. Characterization of a double mutant produced from Arabidopsis thaliana T-DNA insertion lines for ETHE1 and sulfurtransferase 1 revealed that an intermediate of the ETHE1 dependent pathway, most likely a persulfide, interferes with amino acid catabolism and induces early senescence.
PMID:27105581 Hofler S et al; Physiol Plant doi: 10.1111/ppl.12454 (2016) (Epub ahead of print)
Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available. Cysteine is typically synthesized in the human body when there is sufficient methionine available. Cysteine exhibits antioxidant properties and participates in redox reactions. Cysteine's antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans as well as other organisms. Glutathione (GSH) typically requires biosynthesis from its constituent amino acids, cysteine, glycine, and glutamic acid, due to its limited systemic availability. Glutamic acid and glycine are readily available in the diets of most industrialized countries, but the availability of cysteine can be the limiting substrate. In human metabolism, cysteine is also involved in the generation of sulfide present in iron-sulfur clusters and nitrogenase by acting as a precursor. In a 1994 report released by five top cigarette companies, cysteine is one of the 599 additives to cigarettes. Its use or purpose, however, is unknown, like most cigarette additives. Its inclusion in cigarettes could offer two benefits: Acting as an expectorant, since smoking increases mucus production in the lungs; and increasing the beneficial antioxidant glutathione (which is diminished in smokers).

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
15
PharmaCompass offers a list of L-Cysteine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right L-Cysteine manufacturer or L-Cysteine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred L-Cysteine manufacturer or L-Cysteine supplier.
PharmaCompass also assists you with knowing the L-Cysteine API Price utilized in the formulation of products. L-Cysteine API Price is not always fixed or binding as the L-Cysteine Price is obtained through a variety of data sources. The L-Cysteine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A L-Cysteine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of L-Cysteine, including repackagers and relabelers. The FDA regulates L-Cysteine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. L-Cysteine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of L-Cysteine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A L-Cysteine supplier is an individual or a company that provides L-Cysteine active pharmaceutical ingredient (API) or L-Cysteine finished formulations upon request. The L-Cysteine suppliers may include L-Cysteine API manufacturers, exporters, distributors and traders.
click here to find a list of L-Cysteine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A L-Cysteine DMF (Drug Master File) is a document detailing the whole manufacturing process of L-Cysteine active pharmaceutical ingredient (API) in detail. Different forms of L-Cysteine DMFs exist exist since differing nations have different regulations, such as L-Cysteine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A L-Cysteine DMF submitted to regulatory agencies in the US is known as a USDMF. L-Cysteine USDMF includes data on L-Cysteine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The L-Cysteine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of L-Cysteine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The L-Cysteine Drug Master File in Japan (L-Cysteine JDMF) empowers L-Cysteine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the L-Cysteine JDMF during the approval evaluation for pharmaceutical products. At the time of L-Cysteine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of L-Cysteine suppliers with JDMF on PharmaCompass.
A L-Cysteine CEP of the European Pharmacopoeia monograph is often referred to as a L-Cysteine Certificate of Suitability (COS). The purpose of a L-Cysteine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of L-Cysteine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of L-Cysteine to their clients by showing that a L-Cysteine CEP has been issued for it. The manufacturer submits a L-Cysteine CEP (COS) as part of the market authorization procedure, and it takes on the role of a L-Cysteine CEP holder for the record. Additionally, the data presented in the L-Cysteine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the L-Cysteine DMF.
A L-Cysteine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. L-Cysteine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of L-Cysteine suppliers with CEP (COS) on PharmaCompass.
L-Cysteine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of L-Cysteine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right L-Cysteine GMP manufacturer or L-Cysteine GMP API supplier for your needs.
A L-Cysteine CoA (Certificate of Analysis) is a formal document that attests to L-Cysteine's compliance with L-Cysteine specifications and serves as a tool for batch-level quality control.
L-Cysteine CoA mostly includes findings from lab analyses of a specific batch. For each L-Cysteine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
L-Cysteine may be tested according to a variety of international standards, such as European Pharmacopoeia (L-Cysteine EP), L-Cysteine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (L-Cysteine USP).
