
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. (r*,r*)-(+-)-2,3-dihydroxybutanedioic Acid, Monoammonium Monosodium Salt
2. Aluminum Tartrate
3. Ammonium Tartrate
4. Calcium Tartrate
5. Calcium Tartrate Tetrahydrate
6. Mn(iii) Tartrate
7. Potassium Tartrate
8. Seignette Salt
9. Sodium Ammonium Tartrate
10. Sodium Potassium Tartrate
11. Sodium Tartrate
12. Stannous Tartrate
13. Tartaric Acid
14. Tartaric Acid, ((r*,r*)-(+-))-isomer
15. Tartaric Acid, (r*,s*)-isomer
16. Tartaric Acid, (r-(r*,r*))-isomer
17. Tartaric Acid, (s-(r*,r*))-isomer
18. Tartaric Acid, Ammonium Sodium Salt, (1:1:1) Salt, (r*,r*)-(+-)-isomer
19. Tartaric Acid, Calcium Salt, (r-r*,r*)-isomer
20. Tartaric Acid, Monoammonium Salt, (r-(r*,r*))-isomer
21. Tartrate
1. (+)-l-tartaric Acid
2. (+)-tartaric Acid
3. 87-69-4
4. L-(+)-tartaric Acid
5. L(+)-tartaric Acid
6. Tartaric Acid
7. (2r,3r)-2,3-dihydroxysuccinic Acid
8. (2r,3r)-2,3-dihydroxybutanedioic Acid
9. (r,r)-tartaric Acid
10. Threaric Acid
11. L-threaric Acid
12. Dextrotartaric Acid
13. Dl-tartaric Acid
14. Natural Tartaric Acid
15. (2r,3r)-(+)-tartaric Acid
16. (+)-(r,r)-tartaric Acid
17. Tartaric Acid, L-
18. Rechtsweinsaeure
19. (2r,3r)-tartaric Acid
20. (2r,3r)-rel-2,3-dihydroxysuccinic Acid
21. Tartrate
22. (r,r)-(+)-tartaric Acid
23. Fema No. 3044
24. 133-37-9
25. Lamb Protein (fungal)
26. Butanedioic Acid, 2,3-dihydroxy- (2r,3r)-
27. (r,r)-tartrate
28. Tartaric Acid (van)
29. Kyselina Vinna [czech]
30. Ins No.334
31. Uvic Acid
32. Chebi:15671
33. (+)-(2r,3r)-tartaric Acid
34. Ins-334
35. Tartaric Acid [usan:jan]
36. Weinsaeure
37. Mfcd00064207
38. Nsc-62778
39. L-tartarate
40. 4j4z8788n8
41. W4888i119h
42. 138508-61-9
43. Butanedioic Acid, 2,3-dihydroxy-, (2r,3r)-rel-
44. 1,2-dihydroxyethane-1,2-dicarboxylic Acid
45. Resolvable Tartaric Acid
46. D-alpha,beta-dihydroxysuccinic Acid
47. E 334
48. E-334
49. L-(+)-tartrate
50. 144814-09-5
51. Kyselina 2,3-dihydroxybutandiova [czech]
52. Ai3-06298
53. (1r,2r)-1,2-dihydroxyethane-1,2-dicarboxylic Acid
54. 2, 3-dihydroxybutanedioic Acid
55. Butanedioic Acid, 2,3-dihydroxy- (2r,3r)-, Homopolymer
56. Kyselina Vinna
57. Tartaric Acid D,l
58. Butanedioic Acid, 2,3-dihydroxy- (r-(r*,r*))-
59. Tartarate
60. 132517-61-4
61. (+/-)-tartaric Acid
62. Succinic Acid, 2,3-dihydroxy
63. L(+) Tartaric Acid
64. L-2,3-dihydroxybutanedioic Acid
65. (2rs,3rs)-tartaric Acid
66. Einecs 201-766-0
67. Nsc 62778
68. Weinsteinsaeure
69. Weinsaure
70. Tartaric-acid
71. L-threaric Aci
72. Unii-w4888i119h
73. Kyselina 2,3-dihydroxybutandiova
74. 4ebt
75. Nsc 148314
76. Nsc-148314
77. (r,r)-tartarate
78. (2r,3r)-2,3-dihydroxybernsteinsaeure
79. (+)-tartarate
80. (+)-weinsaeure
81. L(+)tartaric Acid
82. Tartaric Acid; L-(+)-tartaric Acid
83. Tartaric Acid (tn)
84. (+-)-tartaric Acid
85. Butanedioic Acid, 2,3-dihydroxy-, (r*,r*)-
86. L-(+) Tartaric Acid
87. (2r,3r)-tartarate
88. 1d5r
89. Dl Tartaric Acid
90. Tartaricum Acidum
91. 2,3-dihydroxy-succinate
92. Tartaric Acid,dl-
93. Dsstox_cid_3632
94. Ec 201-766-0
95. Schembl5762
96. Tartaric Acid [ii]
97. Tartaric Acid, Dl-
98. Dsstox_rid_77120
99. Tartaric Acid (jp17/nf)
100. Tartaric Acid [fcc]
101. Tartaric Acid [jan]
102. D-a,b-dihydroxysuccinic Acid
103. Dsstox_gsid_23632
104. Tartaric Acid [inci]
105. Mls001336057
106. L-tartaric Acid [mi]
107. Tartaric Acid [vandf]
108. Dl-tartaric Acid [mi]
109. Tartaric Acid [mart.]
110. Ccris 8978
111. L-(+)-tartaric Acid, Acs
112. Tartaric Acid [usp-rs]
113. Tartaric Acid [who-dd]
114. Chembl1236315
115. Dtxsid8023632
116. L-(+)-tartaric Acid, Bioxtra
117. Tartaricum Acidum [hpus]
118. Unii-4j4z8788n8
119. (2r,3r)-2,3-tartaric Acid
120. Tartaric Acid (l(+)-)
121. Hms2270g22
122. Pharmakon1600-01300044
123. Tartaric Acid, Dl- [ii]
124. Zinc895301
125. Tartaric Acid, (+/-)-
126. Tartaric Acid,dl- [vandf]
127. Hy-y0293
128. Str02377
129. Tartaric Acid [orange Book]
130. Baros Component Tartaric Acid
131. Einecs 205-105-7
132. Tox21_300155
133. (2r,3r)-2,3-dihydroxysuccinicacid
134. Nsc759609
135. S6233
136. Tartaric Acid [ep Monograph]
137. L-2,3-dihydroxysuccinic Acid
138. Akos016843282
139. L-(+)-tartaric Acid, >=99.5%
140. Cs-w020107
141. Db09459
142. Nsc-759609
143. (2r,3r)-2,3-dihydroxy-succinic Acid
144. Butanedioic Acid, 2,3-dihydroxy-; Butanedioic Acid, 2,3-dihydroxy-, (r-(r*,r*))-
145. Cas-87-69-4
146. L-(+)-tartaric Acid, Ar, >=99%
147. Tartaric Acid Component Of Baros
148. Ncgc00247911-01
149. Ncgc00254043-01
150. Bp-31012
151. Smr000112492
152. Sbi-0207063.p001
153. T0025
154. C00898
155. D00103
156. D70248
157. Dl-tartaric Acid 100 Microg/ml In Acetonitrile
158. L-(+)-tartaric Acid, >=99.7%, Fcc, Fg
159. L-(+)-tartaric Acid, Acs Reagent, >=99.5%
160. L-(+)-tartaric Acid, Bioultra, >=99.5% (t)
161. J-500964
162. J-520420
163. L-(+)-tartaric Acid, Reagentplus(r), >=99.5%
164. L-(+)-tartaric Acid, Saj First Grade, >=99.5%
165. L-(+)-tartaric Acid, Tested According To Ph.eur.
166. Rel-(2r,3r)-2,3-dihydroxybutanedioic Acid
167. Butanedioic Acid, 2,3-dihydroxy-, (r*,r*)-(+-)-
168. L-(+)-tartaric Acid, Jis Special Grade, >=99.5%
169. L-(+)-tartaric Acid, Natural, >=99.7%, Fcc, Fg
170. L-(+)-tartaric Acid, P.a., Acs Reagent, 99.0%
171. L-(+)-tartaric Acid, Vetec(tm) Reagent Grade, 99%
172. Q18226455
173. F8880-9012
174. Z1262250859
175. Butanedioic Acid, 2,3-dihydroxy-, (r-(r*,r*))-
176. Butanedioic Acid, 2,3-dihydroxy-, (theta,theta)-(+-)-
177. 000189e3-11d0-4b0a-8c7b-31e02a48a51f
178. L-(+)-tartaric Acid, Puriss. P.a., Acs Reagent, >=99.5%
179. L-(+)-tartaric Acid, Certified Reference Material, Tracecert(r)
180. Tartaric Acid, United States Pharmacopeia (usp) Reference Standard
181. L-(+)-tartaric Acid, Anhydrous, Free-flowing, Redi-dri(tm), Acs Reagent, >=99.5%
182. L-(+)-tartaric Acid, P.a., Acs Reagent, Reag. Iso, Reag. Ph. Eur., 99.5%
183. Tartaric Acid, Pharmaceutical Secondary Standard; Certified Reference Material
184. L-(+)-tartaric Acid, Puriss. P.a., Reag. Iso, Reag. Ph. Eur., 99.5-101.0% (calc. To The Dried Substance)
185. L-(+)-tartaric Acid, Puriss., Meets Analytical Specification Of Ph. Eur., Bp, Nf, Fcc, E334, 99.7-100.5% (calc. To The Dried Substance), Grit
186. L-(+)-tartaric Acid, Puriss., Meets Analytical Specification Of Ph. Eur., Nf, 99.7-100.5% (calc. To The Dried Substance), Powder
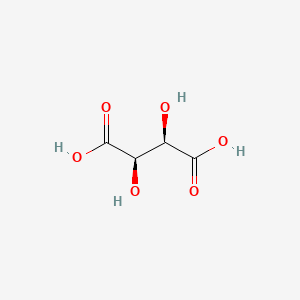
| Molecular Weight | 150.09 g/mol |
|---|---|
| Molecular Formula | C4H6O6 |
| XLogP3 | -1.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 150.01643791 g/mol |
| Monoisotopic Mass | 150.01643791 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 134 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tartaric Acid is primarily indicated in conditions like Antiscorbutic, Antiseptic.
Stress incontinence, female
Tartaric acid is used to generate carbon dioxide through interaction with sodium bicarbonate following oral administration. Carbon dioxide extends the stomach and provides a negative contrast medium during double contrast radiography. In high doses, this agent acts as a muscle toxin by inhibiting the production of malic acid, which could cause paralysis and maybe death.
Absorption
Oral or parenteral doses of monosodium 14C-L(+)-tartrate (400 mg/kg) are rapidly excreted by rats and a proportion completely metabolized to CO2. The oral dose was well-absorbed.
Route of Elimination
Only about 15-20% of consumed tartaric acid is secreted in the urine unchanged.
Most tartarate that is consumed by humans is metabolized by bacteria in the gastrointestinal tract , primarily in the large instestine.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
15
PharmaCompass offers a list of L-Tartaric Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right L-Tartaric Acid manufacturer or L-Tartaric Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred L-Tartaric Acid manufacturer or L-Tartaric Acid supplier.
PharmaCompass also assists you with knowing the L-Tartaric Acid API Price utilized in the formulation of products. L-Tartaric Acid API Price is not always fixed or binding as the L-Tartaric Acid Price is obtained through a variety of data sources. The L-Tartaric Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A L-Tartaric Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of L-Tartaric Acid, including repackagers and relabelers. The FDA regulates L-Tartaric Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. L-Tartaric Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of L-Tartaric Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A L-Tartaric Acid supplier is an individual or a company that provides L-Tartaric Acid active pharmaceutical ingredient (API) or L-Tartaric Acid finished formulations upon request. The L-Tartaric Acid suppliers may include L-Tartaric Acid API manufacturers, exporters, distributors and traders.
click here to find a list of L-Tartaric Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A L-Tartaric Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of L-Tartaric Acid active pharmaceutical ingredient (API) in detail. Different forms of L-Tartaric Acid DMFs exist exist since differing nations have different regulations, such as L-Tartaric Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A L-Tartaric Acid DMF submitted to regulatory agencies in the US is known as a USDMF. L-Tartaric Acid USDMF includes data on L-Tartaric Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The L-Tartaric Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of L-Tartaric Acid suppliers with USDMF on PharmaCompass.
L-Tartaric Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of L-Tartaric Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right L-Tartaric Acid GMP manufacturer or L-Tartaric Acid GMP API supplier for your needs.
A L-Tartaric Acid CoA (Certificate of Analysis) is a formal document that attests to L-Tartaric Acid's compliance with L-Tartaric Acid specifications and serves as a tool for batch-level quality control.
L-Tartaric Acid CoA mostly includes findings from lab analyses of a specific batch. For each L-Tartaric Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
L-Tartaric Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (L-Tartaric Acid EP), L-Tartaric Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (L-Tartaric Acid USP).
