
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
Europe

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
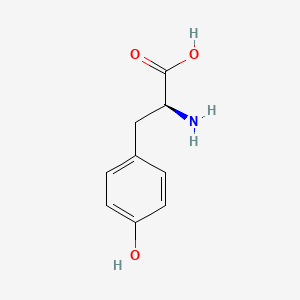

1. L Tyrosine
2. L-tyrosine
3. Para Tyrosine
4. Para-tyrosine
5. Tyrosine, L Isomer
6. Tyrosine, L-isomer
1. L-tyrosine
2. 60-18-4
3. (s)-tyrosine
4. P-tyrosine
5. L-p-tyrosine
6. (2s)-2-amino-3-(4-hydroxyphenyl)propanoic Acid
7. H-tyr-oh
8. 4-hydroxy-l-phenylalanine
9. Tyrosine, L-
10. Tyrosinum [latin]
11. Tyrosine (van)
12. Tirosina [spanish]
13. L-(-)-tyrosine
14. (-)-alpha-amino-p-hydroxyhydrocinnamic Acid
15. L-phenylalanine, 4-hydroxy-
16. Beta-(p-hydroxyphenyl)alanine
17. Tyrosine [usan:inn]
18. Fema No. 3736
19. (s)-alpha-amino-4-hydroxybenzenepropanoic Acid
20. L-2-amino-3-p-hydroxyphenylpropanoic Acid
21. 3-(4-hydroxyphenyl)-l-alanine
22. (s)-3-(p-hydroxyphenyl)alanine
23. (s)-(-)-tyrosine
24. Hsdb 2003
25. Alpha-amino-beta-(4-hydroxyphenyl)propionic Acid
26. Ai3-09055
27. Tirosina
28. Propanoic Acid, 2-amino-3-(4-hydroxyphenyl)-, (s)-
29. Nsc 82624
30. Alpha-amino-p-hydroxyhydrocinnamic Acid, (-)-
31. L-tyr
32. (s)-2-amino-3-(4-hydroxyphenyl)propanoic Acid
33. (s)-2-amino-3-(p-hydroxyphenyl)propionic Acid
34. L-tyrosine, Monomer
35. Alpha-amino-4-hydroxybenzenepropanoic Acid, (s)-
36. 2-amino-3-(4-hydroxyphenyl)propanoic Acid, (s)-
37. Benzenepropanoic Acid, Alpha-amino-4-hydroxy-, (s)-
38. Mfcd00002606
39. Tyr
40. 25619-78-7
41. Chebi:17895
42. Nsc-82624
43. 42hk56048u
44. Ncgc00159350-02
45. Tyrosine (l-tyrosine)
46. Tyrosinum
47. Dsstox_cid_3730
48. L-tyrosin
49. (-)-.alpha.-amino-p-hydroxyhydrocinnamic Acid
50. Dsstox_rid_77170
51. Dsstox_gsid_23730
52. L-tyrosine, Homopolymer
53. Cas-60-18-4
54. L-tyrosine (9ci)
55. 4ts1
56. Tyrosine (usp/inn)
57. Einecs 200-460-4
58. Plovamer-acetate
59. Benzenepropanoate
60. Unii-42hk56048u
61. 2csm
62. (s)-2-amino-3-(4-hydroxyphenyl)propionic Acid
63. (l)-tyrosine
64. (-) Tyrosine
65. H-tyr
66. L-tyrosine,(s)
67. L-tyrosine (jp17)
68. Tyrosine [hsdb]
69. Tyrosine [inci]
70. Tyrosine [usan]
71. Tyrosine [inn]
72. Tyrosine [ii]
73. Tyrosine [mi]
74. L-tyr-oh
75. Tyrosine [vandf]
76. Tyrosine, L- (8ci)
77. .alpha.-amino-.beta.-(4-hydroxyphenyl)propionic Acid
78. L-tyrosine [fcc]
79. L-tyrosine [jan]
80. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase For 6 Hrs
81. Tyrosine [mart.]
82. Bmse000051
83. Chembl925
84. L-tyrosine [fhfi]
85. Tyrosine [who-dd]
86. Schembl1581
87. L-[u-14c]tyr
88. L-phenylalanine-4-hydroxy-
89. L-tyrosine Non-animal Source
90. L-tyrosine [usp-rs]
91. Levodopa Impurity, L-tyrosine-
92. Tyrosine [orange Book]
93. Gtpl4791
94. L-tyrosine, >=97%, Fg
95. Tyrosine [ep Monograph]
96. Dd69927c-c6a8-4bc6-8e9a-0ab423b176e7
97. Dtxsid1023730
98. Tyrosine [usp Monograph]
99. Bdbm18129
100. Zinc266964
101. N-acetyl-o-(dihydroxymethylsilyl)-
102. Hy-n0473
103. L-tyrosine, Vetec(tm), 98.5%
104. Levodopa Related Compound L-tyrosine
105. Tox21_111594
106. (-)-a-amino-p-hydroxyhydrocinnamate
107. Ac2634
108. S4608
109. Akos010400205
110. Tox21_111594_1
111. (s)-a-amino-4-hydroxybenzenepropanoate
112. Am82304
113. Cs-8013
114. Db00135
115. (-)-alpha-amino-p-hydroxyhydrocinnamate
116. (-)-a-amino-p-hydroxyhydrocinnamic Acid
117. (s)-3-(4-hydroxyphenyl)alanine
118. (s)-a-amino-4-hydroxy-benzenepropanoate
119. Ncgc00159350-03
120. Ncgc00344525-01
121. Ac-11295
122. As-11772
123. Bp-13285
124. Levodopa Impurity B [ep Impurity]
125. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase For 6 Hrs, Oxidized With Hydrogen Peroxide
126. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase For 6 Hrs, Oxidized With Hydrogen Peroxide, <3 Kd Fraction
127. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase, Brominated With N-bromosuccinimide
128. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase, Sulfonated Using Sulfur Trioxide/dmf Complex For 1.5-7 Hours
129. (s)-alpha-amino-4-hydroxybenzenepropanoate
130. Diethyl1,3,5-benzenetricarboxylate
131. L-tyrosine, Bioultra, >=99.0% (nt)
132. (s)-2-amino-3-(p-hydroxyphenyl)propionate
133. (s)-a-amino-4-hydroxybenzenepropanoic Acid
134. Db-029987
135. L-tyrosine, Free Base - Cas 60-18-4
136. (s)-a-amino-4-hydroxy-benzenepropanoic Acid
137. (s)-alpha-amino-4-hydroxy-benzenepropanoate
138. L-tyrosine, Reagent Grade, >=98% (hplc)
139. L-tyrosine, Saj Special Grade, >=99.0%
140. T0550
141. C00082
142. D00022
143. D70837
144. L-tyrosine, Vetec(tm) Reagent Grade, >=98%
145. M02963
146. (2s)-2-amino-3-(4-hydroxyphenyl)propanoicacid
147. (s)-alpha-amino-4-hydroxy-benzenepropanoic Acid
148. L-tyrosine, Cell Culture Reagent (h-l-tyr-oh)
149. (s)-.alpha.-amino-4-hydroxybenzenepropanoic Acid
150. 002t606
151. 2-amino-3-(4-hydroxyphenyl)propanoic Acid-(s)-
152. A832631
153. N-acetyltyrosine Impurity A [ep Impurity]
154. Q188017
155. J-521656
156. Propanoic Acid, 2-amino-3-(4-hydroxyphenyl)-(s)-
157. Levodopa Impurity, L-tyrosine- [usp Impurity]
158. Q27115106
159. Benzenepropanoic Acid, .alpha.-amino-4-hydroxy-, (s)-
160. F8889-8713
161. L-tyrosine, Certified Reference Material, Tracecert(r)
162. Tyrosine, European Pharmacopoeia (ep) Reference Standard
163. Z1250208672
164. L-tyrosine, United States Pharmacopeia (usp) Reference Standard
165. 2-amino-3-(4-hydroxyphen Yl)-2-amino-3-(4-hydroxyphenyl)-propanoate
166. 2-amino-3-(4-hydroxyphen Yl)-2-amino-3-(4-hydroxyphenyl)-propanoic Acid
167. Benzeneethanaminium,a-carboxy-4-hydroxy-n,n,n-trimethyl-,inner Salt,(as)-
168. Benzeneethanaminium, A-carboxy-4-hydroxy-n,n,n-trimethyl-,inner Salt, (as)-
169. 1189756-47-5
170. L-tyrosine, From Non-animal Source, Meets Ep, Usp Testing Specifications, Suitable For Cell Culture, >=99.0%
171. L-tyrosine, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 181.19 g/mol |
|---|---|
| Molecular Formula | C9H11NO3 |
| XLogP3 | -2.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 181.07389321 g/mol |
| Monoisotopic Mass | 181.07389321 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 176 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tyrosine is claimed to act as an effective antidepressant, however results are mixed. Tyrosine has also been claimed to reduce stress and combat narcolepsy and chronic fatigue, however these claims have been refuted by some studies.
Tyrosine is a nonessential amino acid synthesized in the body from phenylalanine. Tyrosine is critical for the production of the body's proteins, enzymes and muscle tissue. Tyrosine is a precursor to the neurotransmitters norepinephrine and dopamine. It can act as a mood elevator and an anti-depressant. It may improve memory and increase mental alertness. Tyrosine aids in the production of melanin and plays a critical role in the production of thyroxin (thyroid hormones). Tyrosine deficiencies are manifested by hypothyroidism, low blood pressure and low body temperature. Supplemental tyrosine has been used to reduce stress and combat narcolepsy and chronic fatigue.
Absorption
L-tyrosine is absorbed from the small intestine by a sodium-dependent active transport process.
Semi-chronic exposure of ICR male Mice to Aflatoxin B1 in non-toxic doses results in elevated lung tryptophan levels without change in serotonin or 5-hydroxyindole-3-acetic acid levels. This change is organ specific in that tryptophan levels are not altered in spleen, duodenum, heart or central nervous system. Acute (48 hr) flunixin treatment decreases lung tryptophan levels and reverses the Aflatoxin B1 mediated increase in lung tryptophan levels. On the other hand, flunixin treatment decreases central nervous system tryptophan levels in control mice but not in Aflatoxin B1 treated mice. Aflatoxin B1 treated mice have an increase in splenic serotonin content. Acute (48 hr) treatment of mice with E. coli lipopolysaccharide also increases splenic serotonin, and Aflatoxin B1 treatment followed by lipopolysaccharide have a slightly additive effect on spleen serotonin content. Treatment of mice with lipopolysaccharide increases heart serotonin, an effect which is not altered in Aflatoxin B1 pretreated mice. Both lipopolysaccharide and Aflatoxin B1 per se increases lung tyrosine levels although the combination of treatments is not significantly different from the control value. Flunixin treatment increases lung tyrosine levels, an effect which is not altered by Aflatoxin B1 pretreatment. Acute treatment with either lipopolysaccharide or flunixin decreases the central nervous system tryptophan/tyrosine ratio; pretreatment with Aflatoxin B1 prevents those changes in the central nervous system tryptophan/tyrosine ratio. Central nervous system catecholamines are reduced in Aflatoxin B1 pretreated mice. However, central nervous system catecholamine changes in Aflatoxin B1 treated mice are normalized by vitamin E supplementation during the treatment period.
PMID:1906975 Weekley LB; Metab Brain Dis 6 (1): 19-32 (1991)
Male Wistar rats were divided in free choice conditions into heavy-drinkers consuming greater than 3.5 g/kg of ethanol daily, and light-drinkers consuming less than 2.0 g/kg/day. Subsequent 30 day intragastric administration of 25% ethanol (8-11 g/kg/day) caused an increase in permeability of the blood brain barrier to 14(C)-tyrosine, 14(C)-tryptophan and 14(C)-DOPA at all the stages of alcoholization. All the changes were more pronounced in light-drinkers than in heavy- drinker rats. Disulfiram, and to a lesser extent phenazepam and diazepam, when repeatedly injected (for 16-30 days) together with ethanol aggravated its effects.
PMID:2118321 Borisenko SA; Ann Ist Super Sanita 26 (1): 39-42 (1990)
Effects of mercury chloride (100 uM) para-chloromercuribenzene sulfonate (1 uM), and oxophenylarsine (250 uM) were determined on (a) the rate of sodium pump activity in intact winter flounder intestine; (b) activity of sodium potassium ATPase in tissue homogenates; and (c) sodium-dependent and sodium independent uptake of tyrosine in brush border membrane vesicles. All three agents decreased cell potassium, although effects on cell potassium lagged behind those for inhibition of the ATPase. At the concentrations used in the Ussing chamber (or at one-tenth concentration), all agents completely inhibited sodium potassium ATPase activity in enzyme assays performed with tissue homogenates. In contrast, only mercury chloride decreased sodium dependent uptake of tyrosine by brush border membrane vesicles. These results suggest that mercurial and arsenical effects on tyrosine absorption are due to inhibition of the sodium potassium ATPase thus decreasing the driving force for the cellular uptake by the sodium tyrosine cotransport system. Direct effects on sodium tyrosine cotransport may play a role in the inhibition observed with mercury chloride, but not for para-chloromercuribenzene sulfonate or oxophenylarsine.
PMID:2163123 Musch MW et al; Toxicol Appl Pharmacol 104 (1): 59-66 (1990)
Female Sprague-Dawley rats were treated acutely (12-hr) with aflatoxin B1 (100 ug/kg ip) or vehicle (10% acetone in 0.9% sodium chloride) and regional brain levels of tryptophan, serotonin and tyrosine were assayed. Brainstem but not cerebellar or cortical tyrosine levels were decreased in aflatoxin B1-treated rats. Brain tryptophan was increased in all 3 brain regions by acute aflatoxin B1 treatment, while serotonin levels were unaltered in the cerebellum and cortex and decreased in the brainstem. These experiments indicate that acute aflatoxin B1 treatment differentially alters brain amino acids and serotonin and that changes in brain tryptophan, the serotonin precursor, do not parallel changes in brain serotonin.
PMID:2500755 Weekley LB et al; Toxicol Lett 47 (2): 173-7 (1989)
For more Absorption, Distribution and Excretion (Complete) data for L-TYROSINE (10 total), please visit the HSDB record page.
In the liver, L-tyrosine is involved in a number of biochemical reactions, including protein synthesis and oxidative catabolic reactions. L-tyrosine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body.
/METABOLIC PATHWAY FOR L-TYROSINE:/ /TYROSINE GIVES/ P-HYDROXYPHENYLPYRUVIC ACID GIVES CO2 + HOMOGENTISIC ACID GIVES MALEYLACETOACETIC ACID GIVES FUMARYLACETOACETIC ACID GIVES FUMARATE + ACETOACETATE; TYROSINE GIVES 3,4-DIHYDROXYPHENYLALANINE GIVES CO2 + 3,4-DIHYDROXYPHENYLETHYLAMINE GIVES NORADRENALIN GIVES ADRENALIN.
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 832
L-TYROSINE GIVES N-ACETYL-L-TYROSINE IN MAN; GIVES 3-CARBOXY-L-TYROSINE IN RESEDA; GIVES P-COUMARIC ACID IN SUGAR CANE, L-TYROSINE GIVES PARA-CRESOL IN PROTEUS; GIVES 3,4-DIHYDROXY-L-PHENYLALANINE IN HAMSTER; GIVES 3,4-DIHYDROXYSTILBENE-2-CARBOXYLIC ACID IN HYDRANGEA, L-TYROSINE GIVES 2,7-DIMETHYLNAPHTHOQUINONE IN CHIMAPHILA; GIVES L-DITYROSINE IN BEEF; GIVES PARA-HYDROXYMANDELONITRILE IN SORGHUM, L-TYROSINE GIVES PARA-HYDROXYPHENYLACETALDOXIME IN AUBRETIA; GIVES PARA-HYDROXYPHENYLPYRUVIC ACID IN RAT; GIVES 3-IODO-L-TYROSINE IN BEEF; L-TYROSINE GIVES LACHNANTHOSIDE IN LACHNANTHES; LOPHOCERINE IN LOPHOCERUS; MESEMBRINE IN SCELETIUM; NARWEDINE IN DAFFODIL, L-TYROSINE GIVES NOVOBIOCIN IN STREPTOMYCES; PHENOL IN RAT; BETA-TOCOPHEROL IN ANABAENA; TYLOPHORINE IN TYLOPHORA, L-TYROSINE GIVES TYRAMINE IN RAT; GIVES BETA-TYROSINE IN BACILLUS; GIVES L-TYROSINE HYDROXAMATE IN BEEF. L-TYROSINE GIVES L-TYROSINE-4-PHOSPHATE IN FLY; GIVES XANTHOCILLIN IN PENICILLIUM. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. T-47
Metabolism of tyrosine was impaired after chronic alcoholization of rats with 10% ethanol within 10 months. Within the first 3-4 months activation of tyrosine aminotransferase and a decrease in phenylalanine hydroxylase activity were found in liver tissue. Activity of tyrosine aminotransferase was not increased during the long term alcohol intoxication. At the same time, activity of tyrosine aminotransferase was decreased within 5-6 months simultaneously with activation of phenylalanine hydroxylase. An increase in the alcohol dehydrogenase activity was also observed in rat liver tissue during the initial period of intoxication. The enzymatic activity was decreased beginning from the 3-4 months of the alcoholization and maintained at the low level. Hyperthermia augmented these alterations observed in chronic alcoholization of rats.
Kurbanov KhK, Romakh ON; Vopr Med Khim 35 (4): 102-5 (1989)
Spontaneous behavior subsequent to acute oral administration of high doses of aspartame, phenylalanine, or tyrosine was analyzed using a computer pattern recognition system. Spraque Dawley male rats (250-300 g) were dosed orally with aspartame (500 or 100 mg/kg), phenylalanine (281 or 562 mg/kg), or tyrosine (309 or 618 mg/kg), and their behavior was analyzed 1 hr after dosing. The computer pattern recognition system recorded and classifed 13 different behavioral acts performed by the animals during the first 15-min exploration of a novel environment. These doses of aspartame, phenylalanine, and tyrosine did not induce any significant changes in spontaneous behavior. Unlike low doses of amphetamine and despite high plasma concentrations of phenylalanine and tyrosine, no behavioral alteration was detected by the computer pattern recognition system.
PMID:1677339 Mullenix PJ et al; Fundam Appl Toxicol 16 (3): 495-505 (1991)
For more Metabolism/Metabolites (Complete) data for L-TYROSINE (7 total), please visit the HSDB record page.
Tyrosine is produced in cells by hydroxylating the essential amino acid phenylalanine. This relationship is much like that between cysteine and methionine. Half of the phenylalanine required goes into the production of tyrosine; if the diet is rich in tyrosine itself, the requirements for phenylalanine are reduced by about 50%. The mechanism of L-tyrosine's antidepressant activity can be accounted for by the precursor role of L-tyrosine in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects.
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
31
PharmaCompass offers a list of L-Tyrosine API API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right L-Tyrosine API manufacturer or L-Tyrosine API supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred L-Tyrosine API manufacturer or L-Tyrosine API supplier.
PharmaCompass also assists you with knowing the L-Tyrosine API API Price utilized in the formulation of products. L-Tyrosine API API Price is not always fixed or binding as the L-Tyrosine API Price is obtained through a variety of data sources. The L-Tyrosine API Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A L-Tyrosine API manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of L-Tyrosine API, including repackagers and relabelers. The FDA regulates L-Tyrosine API manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. L-Tyrosine API API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of L-Tyrosine API manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A L-Tyrosine API supplier is an individual or a company that provides L-Tyrosine API active pharmaceutical ingredient (API) or L-Tyrosine API finished formulations upon request. The L-Tyrosine API suppliers may include L-Tyrosine API API manufacturers, exporters, distributors and traders.
click here to find a list of L-Tyrosine API suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A L-Tyrosine API DMF (Drug Master File) is a document detailing the whole manufacturing process of L-Tyrosine API active pharmaceutical ingredient (API) in detail. Different forms of L-Tyrosine API DMFs exist exist since differing nations have different regulations, such as L-Tyrosine API USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A L-Tyrosine API DMF submitted to regulatory agencies in the US is known as a USDMF. L-Tyrosine API USDMF includes data on L-Tyrosine API's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The L-Tyrosine API USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of L-Tyrosine API suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The L-Tyrosine API Drug Master File in Japan (L-Tyrosine API JDMF) empowers L-Tyrosine API API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the L-Tyrosine API JDMF during the approval evaluation for pharmaceutical products. At the time of L-Tyrosine API JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of L-Tyrosine API suppliers with JDMF on PharmaCompass.
A L-Tyrosine API CEP of the European Pharmacopoeia monograph is often referred to as a L-Tyrosine API Certificate of Suitability (COS). The purpose of a L-Tyrosine API CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of L-Tyrosine API EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of L-Tyrosine API to their clients by showing that a L-Tyrosine API CEP has been issued for it. The manufacturer submits a L-Tyrosine API CEP (COS) as part of the market authorization procedure, and it takes on the role of a L-Tyrosine API CEP holder for the record. Additionally, the data presented in the L-Tyrosine API CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the L-Tyrosine API DMF.
A L-Tyrosine API CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. L-Tyrosine API CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of L-Tyrosine API suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing L-Tyrosine API as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for L-Tyrosine API API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture L-Tyrosine API as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain L-Tyrosine API and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a L-Tyrosine API NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of L-Tyrosine API suppliers with NDC on PharmaCompass.
L-Tyrosine API Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of L-Tyrosine API GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right L-Tyrosine API GMP manufacturer or L-Tyrosine API GMP API supplier for your needs.
A L-Tyrosine API CoA (Certificate of Analysis) is a formal document that attests to L-Tyrosine API's compliance with L-Tyrosine API specifications and serves as a tool for batch-level quality control.
L-Tyrosine API CoA mostly includes findings from lab analyses of a specific batch. For each L-Tyrosine API CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
L-Tyrosine API may be tested according to a variety of international standards, such as European Pharmacopoeia (L-Tyrosine API EP), L-Tyrosine API JP (Japanese Pharmacopeia) and the US Pharmacopoeia (L-Tyrosine API USP).
