
Synopsis
Synopsis
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ah 5158
2. Ah-5158
3. Ah5158
4. Albetol
5. Apo Labetalol
6. Apo-labetalol
7. Apolabetalol
8. Dilevalol
9. Hydrochloride, Labetalol
10. Labetalol
11. Labetalol, (r,r)-isomer
12. Labetolol
13. Normodyne
14. Presolol
15. R,r Labetalol
16. R,r-labetalol
17. Sch 19927
18. Sch-19927
19. Sch19927
20. Trandate
1. 32780-64-6
2. Labetalol Hcl
3. Amipress
4. Presdate
5. Normodyne
6. Trandate
7. Labetalol (hydrochloride)
8. Sch 15719w
9. Ah 5158a
10. Sch-15719w
11. 2-hydroxy-5-(1-hydroxy-2-((4-phenylbutan-2-yl)amino)ethyl)benzamide Hydrochloride
12. Ibidomide Hydrochloride
13. Labetalol Hydrochloride
14. Ah-5158a
15. 1gev3baw9j
16. Nsc-290312
17. 32780-64-6 (hcl)
18. Mls000069666
19. Ah-5158a Hydrochloride
20. Sch-15719w Hydrochloride
21. 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]benzamide Hydrochloride
22. 5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)salicylamide Monohydrochloride
23. Ah-5158 Hydrochloride;sch-15719w
24. Labetalol Hcl (mixture Of Diastereomers)
25. Labrocol
26. Pressalolo
27. Smr000058463
28. Ipolab
29. 2-hydroxy-5-(1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl)benzamide Hcl
30. 2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl]benzamide Hydrochloride
31. 2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl]benzamide;hydrochloride
32. 5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)salicylamide Hydrochloride
33. 5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]salicylamide Hydrochloride
34. 5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]salicylamide Monohydrochloride
35. Benzamide, 2-hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)-, Monohydrochloride
36. Dsstox_cid_24654
37. Dsstox_rid_80378
38. Dsstox_gsid_44654
39. Ccris 1086
40. Sr-01000000209
41. Einecs 251-211-1
42. Unii-1gev3baw9j
43. Nsc 290312
44. 2-hydroxy-5-(1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl)benzamide Hydrochloride
45. 2-hydroxy-5-(1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl)benzamide Hydrochloride
46. Normodyne (tn)
47. Trandate (tn)
48. Prestwick_290
49. Einecs 276-694-6
50. Mfcd00057663
51. Labetalolhydrochloride
52. Labetalol Hydrochloride [usan:usp:jan]
53. Ah-5158 Hydrochloride
54. La.beta.lol Hydrochloride
55. Opera_id_1625
56. Benzamide,monohydrochloride
57. Ncgc00016810-01
58. Salicylamide, Hydrochloride
59. Cas-32780-64-6
60. Salicylamide, 5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)-, Hydrochloride
61. Schembl41230
62. 72487-34-4
63. Mls001148626
64. Mls002222197
65. Spectrum1503243
66. Chebi:6344
67. Chembl1200323
68. Dtxsid0044654
69. Amy8842
70. Hms1568h16
71. Hms1922m13
72. Pharmakon1600-01503243
73. 72487-35-5
74. Bcp13886
75. Ex-a4940
76. Hy-b1108
77. Labetalol Hydrochloride [mi]
78. Tox21_110623
79. Tox21_302725
80. Tox21_500687
81. Ccg-40314
82. Labetalol Hydrochloride (jp17/usp)
83. Labetalol Hydrochloride [jan]
84. Nsc290312
85. Nsc758438
86. S4291
87. Labetalol Hydrochloride [usan]
88. Akos015895575
89. Ac-1389
90. Ah-5158
91. Cs-4706
92. Labetalol Hydrochloride [mart.]
93. Labetalol Hydrochloride [vandf]
94. Lp00687
95. Nc00571
96. Nsc-758438
97. Ss-4219
98. Labetalol Hydrochloride [usp-rs]
99. Labetalol Hydrochloride [who-dd]
100. Ncgc00094044-01
101. Ncgc00094044-02
102. Ncgc00094044-03
103. Ncgc00094044-04
104. Ncgc00094044-05
105. Ncgc00256847-01
106. Ncgc00261372-01
107. (r*,r*)-(1)-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)salicylamide Monohydrochloride
108. Benzamide,2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-, Hydrochloride(1:1)
109. Db-048265
110. Eu-0100687
111. Ft-0630564
112. Ft-0773231
113. Labetalol Hydrochloride [orange Book]
114. Sw196595-3
115. Labetalol Hydrochloride [ep Monograph]
116. D00600
117. Labetalol Hydrochloride [usp Monograph]
118. Labetalol Hydrochloride, >98% (tlc), Powder
119. 780l646
120. Normozide Component Labetalol Hydrochloride
121. Sr-01000000209-2
122. Sr-01000000209-7
123. Labetalol Hydrochloride Component Of Normozide
124. Q27252396
125. 2-hydroxy-5-(1-hydroxy-2-((4-phenylbutan-2-yl)amino)ethyl)benzamide Hcl
126. Labetalol Hydrochloride, European Pharmacopoeia (ep) Reference Standard
127. Labetalol Hydrochloride, United States Pharmacopeia (usp) Reference Standard
128. Labetalol Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
129. Benzamide, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-, Hydrochloride (1:1)
130. Benzamide,2-hydroxy-5-[(1s)-1-hydroxy-2-[[(1r)-1-methyl-3-phenylpropyl]amino]ethyl]-,hydrochloride (1:1), Rel-
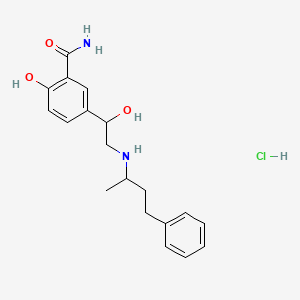
| Molecular Weight | 364.9 g/mol |
|---|---|
| Molecular Formula | C19H25ClN2O3 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 364.1553704 g/mol |
| Monoisotopic Mass | 364.1553704 g/mol |
| Topological Polar Surface Area | 95.6 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 385 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Labetalol hydrochloride |
| Drug Label | Labetalol HCl USP tablets are adrenergic receptor blocking agents that have both selective alpha1-adrenergic and non-selective beta-adrenergic receptor blocking actions in a single substance.Labetalol HCl USP is a racemate, chemically designated as 5... |
| Active Ingredient | Labetalol hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 200mg; 300mg; 5mg/ml; 100mg |
| Market Status | Prescription |
| Company | Hospira; Gland Pharma; Sandoz; Watson Labs; Ivax Sub Teva Pharms; Eurohlth Intl; Par Form; Akorn |
| 2 of 2 | |
|---|---|
| Drug Name | Labetalol hydrochloride |
| Drug Label | Labetalol HCl USP tablets are adrenergic receptor blocking agents that have both selective alpha1-adrenergic and non-selective beta-adrenergic receptor blocking actions in a single substance.Labetalol HCl USP is a racemate, chemically designated as 5... |
| Active Ingredient | Labetalol hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 200mg; 300mg; 5mg/ml; 100mg |
| Market Status | Prescription |
| Company | Hospira; Gland Pharma; Sandoz; Watson Labs; Ivax Sub Teva Pharms; Eurohlth Intl; Par Form; Akorn |
Adrenergic beta-Antagonists
Drugs that bind to but do not activate beta-adrenergic receptors thereby blocking the actions of beta-adrenergic agonists. Adrenergic beta-antagonists are used for treatment of hypertension, cardiac arrhythmias, angina pectoris, glaucoma, migraine headaches, and anxiety. (See all compounds classified as Adrenergic beta-Antagonists.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Adrenergic alpha-1 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC ALPHA-1 RECEPTORS. (See all compounds classified as Adrenergic alpha-1 Receptor Antagonists.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.

GDUFA
DMF Review : Reviewed
Rev. Date : 2017-01-31
Pay. Date : 2017-01-11
DMF Number : 30564
Submission : 2016-06-07
Status : Active
Type : II

Date of Issue : 2022-06-08
Valid Till : 2025-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2025
Start Marketing Date : 2016-07-09
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-03-06
Pay. Date : 2014-03-04
DMF Number : 16314
Submission : 2002-12-17
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23710
Submission : 2010-04-13
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38064
Submission : 2023-03-30
Status : Active
Type : II

NDC Package Code : 66039-962
Start Marketing Date : 2022-08-08
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-09-07
Pay. Date : 2023-07-26
DMF Number : 37306
Submission : 2022-08-09
Status : Active
Type : II

Certificate Number : CEP 2022-315 - Rev 00
Issue Date : 2024-08-23
Type : Chemical
Substance Number : 923
Status : Valid

NDC Package Code : 82245-0203
Start Marketing Date : 1987-04-06
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2017-04-17
Pay. Date : 2017-03-27
DMF Number : 31584
Submission : 2017-03-29
Status : Active
Type : II

NDC Package Code : 53747-037
Start Marketing Date : 2017-03-29
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17319
Submission : 2004-04-13
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?