
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
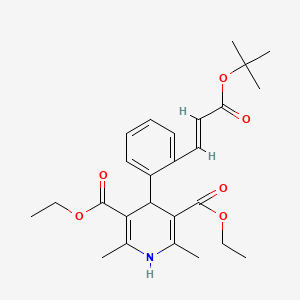

1. Caldine
2. Gr 43659x
3. Gr-43659x
4. Lacimen
5. Lacipil
6. Motens
1. 103890-78-4
2. Lacipil
3. Motens
4. Gr-43659x
5. Trans Lacidipine
6. Gr 43659x
7. Lacidipine (lacipil, Motens)
8. Diethyl 2,6-dimethyl-4-[2-[(e)-3-[(2-methylpropan-2-yl)oxy]-3-oxoprop-1-enyl]phenyl]-1,4-dihydropyridine-3,5-dicarboxylate
9. Gx-1048
10. Sn-305
11. 4-(o-((e)-2-carboxyvinyl)phenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic Acid, 4-tert-butyl Diethyl Ester
12. Lacimen
13. Dsstox_cid_26429
14. Dsstox_rid_81607
15. Dsstox_gsid_46429
16. 3,5-pyridinedicarboxylic Acid, 4-(2-(3-(1,1-dimethylethoxy)-3-oxo-1-propenyl)phenyl)-1,4-dihydro-2,6-dimethyl-, Diethyl Ester, (e)-
17. Diethyl (e)-4-(2-(3-(tert-butoxy)-3-oxoprop-1-en-1-yl)phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
18. 260080034n
19. Lacidipinum [latin]
20. Lacidipino [spanish]
21. Lacidipino
22. Lacidipinum
23. Lacirex
24. (e)-diethyl 4-(2-(3-(tert-butoxy)-3-oxoprop-1-en-1-yl)phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
25. Smr000466342
26. Cas-103890-78-4
27. Sr-05000001445
28. Gr 43659 X
29. Viapres
30. Molap
31. Lacidipine [usan:inn:ban]
32. Lacidipine,(s)
33. Ncgc00164545-01
34. (e)-diethyl 4-(2-(3-tert-butoxy-3-oxoprop-1-enyl)phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
35. Lacidipine- Bio-x
36. Motens (tn)
37. Unii-260080034n
38. Lacidipine [mi]
39. Lacidipine [inn]
40. Lacidipine [jan]
41. Lacidipine (usan/inn)
42. Lacidipine [usan]
43. Lacidipine [vandf]
44. Lacidipine [mart.]
45. Lacidipine [who-dd]
46. Lacipil, Motens, Lacidipine
47. Schembl49277
48. Schembl49278
49. Mls000759454
50. Mls001424282
51. Chembl460291
52. Chembl1728809
53. Dtxsid1046429
54. Schembl13287288
55. Chebi:94480
56. Gtpl11740
57. Chebi:135737
58. Hms2052f03
59. Hms2089k22
60. Hms3713d20
61. Hms3884i18
62. (non-isotopelabelled)lacidipine-d9
63. Bcp02933
64. Gr43659x
65. Hy-b0347
66. Tox21_112174
67. Gx1048
68. S1994
69. Stl454986
70. Akos005066844
71. Tox21_112174_1
72. Zinc100015470
73. Bcp9000831
74. Ccg-101153
75. Ccg-220579
76. Db09236
77. Gx 1048
78. Hs-0086
79. Nc00403
80. Ncgc00263529-01
81. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(2-(3-(1,1-dimethylethoxy)-3-oxo-1-propenyl)phenyl)-, Diethyl Ester, (e)-
82. Ac-11008
83. Ac-33195
84. Bl164603
85. Cpd000466342
86. Bcp0726000285
87. L0276
88. Sw219840-1
89. C71162
90. D04657
91. Ab00698350-05
92. Ab01275445-01
93. Ab01275445_02
94. Gx-1048,gr-43659x,sn-305
95. 890l784
96. A800840
97. J-001058
98. Q1163827
99. Sr-05000001445-1
100. Sr-05000001445-2
101. Brd-k05851096-001-01-9
102. 2,6-dimethyl-4-[2-[(e)-3-[(2-methylpropan-2-yl)oxy]-3-oxoprop-1-enyl]phenyl]-1,4-dihydropyridine-3,5-dicarboxylic Acid Diethyl Ester
103. Diethyl 2,6-dimethyl-4-[2-[(e)-3-[(2-methylpropan-2-yl)oxy]-3-oxidanylidene-prop-1-enyl]phenyl]-1,4-dihydropyridine-3,5-dicarboxylate
104. Diethyl 4-{2-[(1e)-3-tert-butoxy-3-oxoprop-1-en-1-yl]phenyl}-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
| Molecular Weight | 455.5 g/mol |
|---|---|
| Molecular Formula | C26H33NO6 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 455.23078777 g/mol |
| Monoisotopic Mass | 455.23078777 g/mol |
| Topological Polar Surface Area | 90.9 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 805 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of hypertension either alone or in combination with other antihypertensive agents, including -adrenoceptor antagonists, diuretics, and ACE-inhibitors.
acidipine is a specific and potent calcium antagonist with a predominant selectivity for calcium channels in the vascular smooth muscle. Its main action is to dilate predominantly peripheral and coronary arteries, reducing peripheral vascular resistance and lowering blood pressure. Following the oral administration of 4 mg lacidipine to volunteer subjects, a minimal prolongation of QTc interval has been observed (mean QTcF increase between 3.44 and 9.60 ms in young and elderly volunteers).
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA09 - Lacidipine
Absorption
Since it is a highly lipophilic compound, lacidpine is rapidly absorbed from the gastrointestinal tract following oral administration with the peak plasma concentrations reached between 30 and 150 minutes of dosing. The peak plasma concentrations display large interindividual variability, with the values ranging from 1.6 to 5.7 g/L following single-dose oral administration of lacidipine 4mg in healthy young volunteers. Absolute bioavailability is less than 10% due to extensive first-pass metabolism in the liver.
Route of Elimination
Approximately 70% of the administered dose is eliminated as metabolites in the faeces and the remainder as metabolites in the urine.
Lacidipine undergoes complete CYP3A4-mediated hepatic metabolism, with no parent drug detected in the urine or faeces. The 2 main metabolites have no pharmacological activity.
The average terminal half-life of lacidipine ranges from between 13 and 19 hours at steady state.
By blocking the voltage-dependent L-type calcium channels, it prevents the transmembrane calcium influx. Normally, calcium ions serve as intracellular messengers or activators in exictable cells including vascular smooth muscles. The influx of calcium ultimately causes the excitation and depolarization of the tissues. Lacidipine inhibits the contractile function in the vascular smooth muscle and reduce blood pressure. Due to its high membrane partition coefficient, some studies suggest that lacidipine may reach the receptor via a two-step process; it first binds and accumulates in the membrane lipid bilayer and then diffuses within the membrane to the calcium channel receptor. It is proposed that lacidipine preferentially blocks the inactivated state of the calcium channel. Through its antioxidant properties shared amongst other dihydropyridine calcium channel blockers, lacidipine demonstrates an additional clinical benefit. Its antiatherosclerotic effects are mediated by suppressing the formation of reactive oxygen species (ROS) and subsequent inflammatory actions by chemokines, cytokines and adhesion molecules, thus reducing atherosclerotic lesion formation. Lacidipine may also suppress cell proliferation and migration in smooth muscle cells and suppress the expression of matrix metalloproteinases, which affects the stability of atheromatous plaques.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
82
PharmaCompass offers a list of Lacidipine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lacidipine manufacturer or Lacidipine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lacidipine manufacturer or Lacidipine supplier.
PharmaCompass also assists you with knowing the Lacidipine API Price utilized in the formulation of products. Lacidipine API Price is not always fixed or binding as the Lacidipine Price is obtained through a variety of data sources. The Lacidipine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lacidipine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lacidipine, including repackagers and relabelers. The FDA regulates Lacidipine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lacidipine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lacidipine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lacidipine supplier is an individual or a company that provides Lacidipine active pharmaceutical ingredient (API) or Lacidipine finished formulations upon request. The Lacidipine suppliers may include Lacidipine API manufacturers, exporters, distributors and traders.
click here to find a list of Lacidipine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lacidipine DMF (Drug Master File) is a document detailing the whole manufacturing process of Lacidipine active pharmaceutical ingredient (API) in detail. Different forms of Lacidipine DMFs exist exist since differing nations have different regulations, such as Lacidipine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lacidipine DMF submitted to regulatory agencies in the US is known as a USDMF. Lacidipine USDMF includes data on Lacidipine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lacidipine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lacidipine suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lacidipine Drug Master File in Korea (Lacidipine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lacidipine. The MFDS reviews the Lacidipine KDMF as part of the drug registration process and uses the information provided in the Lacidipine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lacidipine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lacidipine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lacidipine suppliers with KDMF on PharmaCompass.
A Lacidipine written confirmation (Lacidipine WC) is an official document issued by a regulatory agency to a Lacidipine manufacturer, verifying that the manufacturing facility of a Lacidipine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lacidipine APIs or Lacidipine finished pharmaceutical products to another nation, regulatory agencies frequently require a Lacidipine WC (written confirmation) as part of the regulatory process.
click here to find a list of Lacidipine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lacidipine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lacidipine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lacidipine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lacidipine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lacidipine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lacidipine suppliers with NDC on PharmaCompass.
Lacidipine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lacidipine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lacidipine GMP manufacturer or Lacidipine GMP API supplier for your needs.
A Lacidipine CoA (Certificate of Analysis) is a formal document that attests to Lacidipine's compliance with Lacidipine specifications and serves as a tool for batch-level quality control.
Lacidipine CoA mostly includes findings from lab analyses of a specific batch. For each Lacidipine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lacidipine may be tested according to a variety of international standards, such as European Pharmacopoeia (Lacidipine EP), Lacidipine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lacidipine USP).
