
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
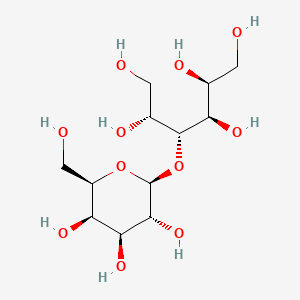

1. 4-o-beta-d-galactopyranosyl-d-glucitol
2. Emportal
3. Importal
4. Neda Lactiv Importal
5. Oponaf
1. 585-86-4
2. Importal
3. 4-o-beta-d-galactopyranosyl-d-glucitol
4. D-lactitol
5. Lactositol
6. Lactosit
7. Miruhen
8. Lactitolum
9. Lactobiosit
10. Lacitol
11. Lactit
12. Milchen
13. Finlac Dc
14. Lactitol Acm 50
15. Lacty (saccharide)
16. Lactitol Anhydrous
17. L2b0wjf7zy
18. Nsc 231323
19. Nsc-231323
20. Ins No.966
21. Chebi:75323
22. Ins-966
23. Lactitol Hydrate
24. D-glucitol, 4-o-beta-d-galactopyranosyl-
25. E-966
26. Dsstox_cid_24247
27. Dsstox_rid_80133
28. Dsstox_gsid_44247
29. Emportal
30. Oponaf
31. Bli-400
32. (2s,3r,4r,5r)-4-(((2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)hexane-1,2,3,5,6-pentaol
33. Cas-585-86-4
34. Nsc-759131
35. Lactitolum [inn-latin]
36. Unii-l2b0wjf7zy
37. Lactitol [inn:ban:nf]
38. Floralac
39. Portolac
40. Ccris 7077
41. Lactosit Miruhen
42. Nsc231323
43. Hsdb 7970
44. Ncgc00166295-01
45. Importal (tn)
46. Einecs 209-566-5
47. Pizensy (tn)
48. Lactitol (nf/inn)
49. Lactitol [inci]
50. Lactitol [inn]
51. Lactitol [mi]
52. Lactitol [usp-rs]
53. Lactitol [who-dd]
54. Schembl3849
55. Chembl1661
56. Lactitol, Analytical Standard
57. Lactitol [orange Book]
58. Dtxsid9044247
59. Hms3264e13
60. Pharmakon1600-01301027
61. Hy-n7104
62. Zinc5225520
63. Tox21_112397
64. Mfcd00079407
65. Nsc760415
66. S5368
67. 4-beta-d-galactopyranosyl-d-glucito1
68. Akos030228540
69. Tox21_112397_1
70. Ccg-213712
71. Db12942
72. Nsc-760415
73. Ncgc00263893-02
74. (2s,3r,4r,5r)-4-[(2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexane-1,2,3,5,6-pentol
75. 4-o-b-d-galactopyranosyl-d-glucitol, 9ci
76. Beta-d-galactopyranosyl-(1->4)-d-glucitol
77. Cs-0069039
78. A11574
79. D08266
80. Ab00698230_06
81. 4-o-.beta.-d-galactopyranosyl-d-glucitol
82. Q415020
83. W-109090
84. Brd-k40787673-001-02-1
85. 262a7827-24f7-47ee-b528-3af52ca860ce
86. Wurcs=2.0/2,2,1/[h2122h][a2112h-1b_1-5]/1-2/a4-b1
87. (2s,3r,4r,5r)-4-((2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yloxy)hexane-1,2,3,5,6-pentaol
88. (2s,3r,4r,5r)-4-[(2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-methylol-tetrahydropyran-2-yl]oxyhexane-1,2,3,5,6-pentol
| Molecular Weight | 344.31 g/mol |
|---|---|
| Molecular Formula | C12H24O11 |
| XLogP3 | -5.2 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 344.13186158 g/mol |
| Monoisotopic Mass | 344.13186158 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sugar Alcohols; Cathartics; Sweetening Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Lactitol (beta-galactosido-sorbitol) has been recently compared with lactulose for the treatment of chronic hepatic encephalopathy in a few studies, each comprising a small number of patients. The results are controversial. We studied the efficiency and tolerance of both compounds by using a meta-analysis on the basis of published controlled trials. /This/ study only included controlled or randomized trials comprising cirrhotic patients with chronic hepatic encephalopathy. Analyzed parameters were the portosystemic encephalopathy index of Conn after treatment, the percentage of improved patients and the percentage of patients who had ill effects related to the treatment (flatulence, diarrhea). Bibliographical screening revealed five studies comparing the effects of lactitol and lactulose in chronic hepatic encephalopathy. Four crossover studies were done that included 48 patients and one parallel study that included 29 patients. The duration of the treatment ranged from 3 to 6 mo. All studies found a similar efficiency with both drugs. However, they exhibited some discrepancies in the relative frequency of adverse reactions (flatulence). Meta-analysis showed no statistical differences in the portosystemic encephalopathy index after lactitol or lactulose treatment. The percentage of improved patients after lactitol or lactulose was similar. In contrast, the analysis revealed a higher frequency (p less than 0.01) of flatulence in patients treated with lactulose compared with those treated with lactitol. In conclusion, this meta-analysis shows no statistical difference between therapeutic effects of lactitol and lactulose, but it does show a higher frequency of flatulence with lactulose. This suggests that lactitol should be preferred to lactulose for the treatment of chronic hepatic encephalopathy.
PMID:1531204 Blanc P et al; Hepatology 15 (2): 222-8 (1992)
Preliminary data suggest that lactitol (beta-galactoside-sorbitol), a new synthetic non-absorbable disaccharide, has beneficial effects on chronic portal systemic encephalopathy. To compare the efficacy of lactitol vs. lactulose in the treatment of acute portal systemic encephalopathy (PSE), 40 cirrhotic patients with an acute episode of PSE were randomly allocated to one of two groups: group A (20 patients) received lactulose (30 mL/6 hr) and group B (20 patients) lactitol (12 g/6 hr). These doses were adjusted daily to obtain two bowel movements per day. The duration of treatment was 5 days. Age, sex, hepatic and renal function, precipitating factors and level of PSE measured by clinical examination, EEG and number connection test were similar in the two groups. A complete clinical resolution of PSE occurred in 11 patients in each group. In 5 patients of the lactulose group and in 6 of the lactitol group there was a moderate improvement of PSE during the study. Finally, 4 patients in the lactulose group and 3 in the lactitol group did not respond to treatment. No side effects attributable to therapy were observed in either group. These results indicate that lactitol is as effective as lactulose in the management of patients with cirrhosis and acute PSE.
PMID:3598162 Heredia D et al; J Hepatol 4 (3): 293-8 (1987)
Lactitol is indicated for the treatment of chronic idiopathic constipation in adults.
FDA Label
Lactitol helps to facilitate bowel movements by drawing water into the gastrointestinal tract. The oral administration of lactitol may reduce the absorption of concomitant medications - other oral medications should be administered at least 2 hours before or 2 hours after lactitol.
Cathartics
Agents that are used to stimulate evacuation of the bowels. (See all compounds classified as Cathartics.)
Sweetening Agents
Substances that sweeten food, beverages, medications, etc., such as sugar, saccharine or other low-calorie synthetic products. (From Random House Unabridged Dictionary, 2d ed) (See all compounds classified as Sweetening Agents.)
A06AD12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AD - Osmotically acting laxatives
A06AD12 - Lactitol
Absorption
In healthy subjects under fed conditions, oral administration of 20 grams of lactitol resulted in a mean Tmax of 3.6 1.2 hours, Cmax of 776 253 ng/mL, and a mean AUC of 6,019 1,771 ng*hr/mL.
Route of Elimination
Lactitol is not absorbed in the gastrointestinal tract to any significant extent. The vast majority of an ingested dose is likely degraded into organic acids in the colon and eliminated in the feces.
Volume of Distribution
Data regarding the volume of distribution of lactitol are unavailable.
Clearance
Data regarding the clearance of lactitol are unavailable.
Three male rats (150-200 g; six to eight weeks of age; one not pretreated and two habituated to a diet containing 7% lactitol) were orally intubated with about 2 mg D-(sorbitol-1-(14)C) lactitol. In the studies with the rats habituated to lactitol, 9-15% of the radioactivity was recovered from the air exhaled in the period 0-5 hours and 48% from the air exhaled in the period 0-24 hours. The urine and the feces contained a minor proportion of the administered radioactivity (urine, 2.3% after five hours and 6.8% after 24 hours; feces, 11.7% after 24 hours). The gastrointestinal tract contained 33% of the radioactivity after five hours and 5% after 24 hours; the remainder of the body contained 20% after five hours and 9% after 24 hours. It was concluded that lactitol is extensively degraded in the rat after oral administration presumably mainly by the intestinal microflora and that habituation of the rats to unlabelled lactitol did not essentially affect the rate and extent of degradation.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 18: Lactitol (585-86-4) (1983). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
In studies designed to investigate the metabolism of erythritol in vivo in healthy volunteers and to compare the fermentation of erythritol by human fecal flora in vitro with that of glucose and lactitol, four male and two female volunteers aged 21-25 undertook an overnight fast and were then chosen at random to receive a single dose of 25 g (13)C-erythritol, (13)C-glucose, and (13)C-lactitol in 250 mL of water with at least three days between each treatment. Breath samples were taken for analysis of (13)C-carbon dioxide and hydrogen gas before treatment and at 30 min intervals up to 6 hr after treatment. The ratio of (13)C: (12)C-carbon dioxide was measured by isotope-ratio mass spectrometry. Urine samples were collected over 0-6 and 6-24 hr after treatment, and the erythritol and lactitol concentrations in urine were measured by HPLC. ... During the first 6 and 24 hr after dosing, 52 and 84%, respectively, of the administered erythritol was recovered in the urine. No increase in expired (13)C-carbon dioxide or hydrogen gas was observed, indicating that no fermentation had occurred in the gut. In contrast, there was a rapid increase in expired (13)C-carbon dioxide after consumption of glucose and a more gradual rise after ingestion of lactitol. Excretion of hydrogen gas in expired air was also increased after treatment with lactitol. Neither lactitol nor glucose was detected in significant amounts in the urine. ...
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
The gastrointestinal absorption of lactitol has been studied in 6 healthy volunteers and 8 patients with cirrhosis. Following administration of lactitol 0.5 g/kg, no lactitol was found in serum. The urinary excretion of lactitol over 24 hr ranged from 0.1 to 1.4% of the administered dose (0.46% in cirrhotics and 0.35% in healthy volunteers). Blood D- and L-lactate and plasma glucose did not increase following lactitol. The data indicate that lactitol was poorly absorbed from the gastrointestinal tract in healthy volunteers and patients with cirrhosis, and that the disaccharide did not disturb glucose or lactate homeostasis.
PMID:3220101 Metzger J et al; Eur J Clin Pharmacol 35 (1): 97-9 (1988)
The fate of orally ingested lactitol, a non-absorbed sugar, was measured in six healthy human subjects by following the three routes of disposal of universally (14)C-labelled sugar. Lactitol was given as a 20 g daily dose to six healthy volunteers for 14 days and on the seventh day, 10 muCi of L-[U-(14)C]-lactitol was given with the unlabelled sugar and excretion of the (14)C in breath, urine and faeces was followed. The peak of (14)CO2 excretion occurred at six hours and total (14)CO2 accounted for 62.9 (5.0)% of isotope given, whilst 6.5 (3.6)% and 2.0 (0.3)% of the label were recovered from faeces and urine respectively. These data suggest that lactitol is extensively metabolised in the human colon and that a significant proportion of the bacterial metabolites are available for colonic absorption. Calculation revealed that 54.5% of the theoretical energy content of this compound was utilised by the subjects. ...
PMID:3220306 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1434111 Grimble GK et al; Gut 29 (12): 1666-71 (1988)
As it undergoes little-to-no systemic absorption, lactitol is unlikely to undergo any significant degree of metabolism.
In studies designed to investigate the metabolism of erythritol in vivo in healthy volunteers and to compare the fermentation of erythritol by human fecal flora in vitro with that of glucose and lactitol, four male and two female volunteers aged 21-25 undertook an overnight fast and were then chosen at random to receive a single dose of 25 g (13)C-erythritol, (13)C-glucose, and (13)C-lactitol in 250 mL of water with at least three days between each treatment. Breath samples were taken for analysis of (13)C-carbon dioxide and hydrogen gas before treatment and at 30 min intervals up to 6 hrs after treatment. The ratio of (13)C: (12)C-carbon dioxide was measured by isotope-ratio mass spectrometry. ... In order to maintain a constant metabolic rate, the subjects remained at rest during the study. For the assay of fermentation in vitro, fecal samples were collected from six healthy volunteers (sex and age not specified) who ate a normal western diet. None of the subjects complained of gastrointestinal symptomsand none had used antibiotics in the past six months. The samples were incubated under anaerobic conditions for 6 hr, and then the hydrogen gas concentration was measured in the head-space of the incubation vials. ... After a 6 hr incubation with erythritol, the amount of hydrogen gas formed by the fecal flora was comparable to that in control vials, but significantly (p < 0.001) more hydrogen gas was produced in the glucose and lactitol vials than in either control or erythritol.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
The average half-life of orally administered lactitol is 2.4 hours.
Lactitol is an osmotic laxative - it exerts its pharmacologic effect by creating a hyperosmotic environment within the small intestine. The osmotic effect generated by lactitol draws water into the small intestine, which loosens stools and ultimately facilitates bowel movements.
Lactitol, an unabsorbed sugar with defined laxative threshold and superior taste properties has been suggested as an alternative to lactulose in the treatment of hepatic encephalopathy. In the present study /investigators/ compared the colonic metabolism of the two sugars using an in vitro fecal incubation system. Both sugars were readily metabolized by fecal bacteria producing volatile fatty acids and the metabolism was inhibited by neomycin. The effect of lactitol and lactulose on terminal ileal and colonic pH was monitored in six normal subjects using a radiotelemetry technique. Both sugars significantly lowered right colonic pH (basal -6.51 +/- 0.48 vs lactitol -5.63 +/- 0.50; lactulose -5.18 +/- 0.82, p less than 0.05). The pH of rest of the colon and terminal ileum was unaffected. Neomycin given concurrently with lactulose abolished acidification of right colon. As lactitol and lactulose have similar effects within the colon, lactitol would appear to have a role in the treatment of hepatic encephalopathy. As neomycin antagonizes the effect of lactulose in the colon, its concurrent use may be less effective in the treatment of hepatic encephalopathy.
PMID:3570029 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1432706 Patil DH et al; Gut 28 (3): 255-9 (1987)
... Reports from authoritative bodies and reviews indicates that the decrease in pH in plaque as a consequence of metabolic acid production by saccharolytic bacteria when exposed to fermentable carbohydrates (i.e. sugars and starches) may promote demineralization and prevent remineralization of the hydroxyapatite crystals. Tooth hydroxyapatite crystals are very resistant to dissolution at neutral pH, but their solubility drastically increases as pH drops. Typically, the critical pH for dental enamel is around 5.5. ... Demineralization of tooth tissues can also occur as a result of consumption of dietary acids in foods or beverages, and that frequent consumption can lead to dental erosion. Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose are slowly metabolized by bacteria in the mouth. The rate and amount of acid production from these food constituents is significantly less than that from sucrose. ... Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose do not promote dental caries because they do not lower plaque pH to the level associated with enamel demineralization. ... A cause and effect relationship has been established between the consumption of sugar-containing foods/drinks at an exposure frequency of four times daily or more and an increased tooth demineralization, and that the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose, instead of sugar in sugar-containing foods/drinks, may maintain tooth mineralization by decreasing tooth demineralization compared with sugar-containing foods, provided that such foods/drinks do not lead to dental erosion.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm
The food constituents xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose resulted in reduced post-prandial blood glucose (or insulinemic) responses compared with sugars on a weight by weight basis owing to their reduced/delayed digestion/absorption and/or to a decrease in the amount of available carbohydrates, and that the consumption of foods/drinks in which xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose replaced sugars induced lower post-prandial glycemic and insulinemic responses than sugar-containing foods/drinks. ... A cause and effect relationship has been established between the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose instead of sugar and reduction in post-prandial blood glucose responses (without disproportionally increasing post-prandial insulinemic responses) as compared to sugar-containing foods/drinks.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm
ABOUT THIS PAGE
73
PharmaCompass offers a list of Lactitolum API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lactitolum manufacturer or Lactitolum supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lactitolum manufacturer or Lactitolum supplier.
PharmaCompass also assists you with knowing the Lactitolum API Price utilized in the formulation of products. Lactitolum API Price is not always fixed or binding as the Lactitolum Price is obtained through a variety of data sources. The Lactitolum Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lactitolum manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lactitolum, including repackagers and relabelers. The FDA regulates Lactitolum manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lactitolum API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Lactitolum supplier is an individual or a company that provides Lactitolum active pharmaceutical ingredient (API) or Lactitolum finished formulations upon request. The Lactitolum suppliers may include Lactitolum API manufacturers, exporters, distributors and traders.
Lactitolum Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lactitolum GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lactitolum GMP manufacturer or Lactitolum GMP API supplier for your needs.
A Lactitolum CoA (Certificate of Analysis) is a formal document that attests to Lactitolum's compliance with Lactitolum specifications and serves as a tool for batch-level quality control.
Lactitolum CoA mostly includes findings from lab analyses of a specific batch. For each Lactitolum CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lactitolum may be tested according to a variety of international standards, such as European Pharmacopoeia (Lactitolum EP), Lactitolum JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lactitolum USP).

