
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
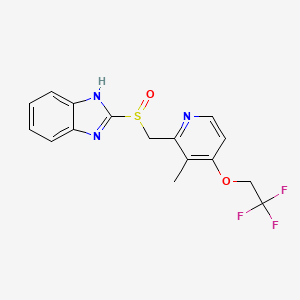

1. 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl)methyl)sulfinyl)benzimidazole
2. Ag 1749
3. Ag-1749
4. Ag1749
5. Agopton
6. Bamalite
7. Lansol
8. Lansoprazol
9. Lansoprazole Sodium
10. Lansoprazoles
11. Lanzor
12. Monolitum
13. Ogast
14. Ogastro
15. Opiren
16. Prevacid
17. Prezal
18. Pro Ulco
19. Promeco
20. Sodium, Lansoprazole
21. Takepron
22. Ulpax
23. Zoton
1. 103577-45-3
2. Prevacid
3. Agopton
4. Limpidex
5. Lanzor
6. Bamalite
7. Monolitum
8. Takepron
9. Ogastro
10. Lansoprazol
11. Opiren
12. Zoton
13. Prevacid Solutab
14. Lanzopral
15. Ag-1749
16. Lanproton
17. Lansopep
18. Lasoprol
19. Mesactol
20. Aprazol
21. Blason
22. Ketian
23. Lancid
24. Lanston
25. Ogast
26. Lanz
27. Lanzoprazole
28. Lansoprazolum
29. Lansox
30. Prevacid Iv
31. Prevonco
32. Ag 1749
33. A-65006
34. Lanzo
35. Prevacid 24hr
36. Ogastoro
37. 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1h-benzimidazole
38. Chebi:6375
39. Prezal
40. Pro Ulco
41. 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl)methyl)sulfinyl)benzimidazole
42. Compraz
43. Ilsatec
44. Prosogan
45. Suprecid
46. Dakar
47. Promp
48. Zoprol
49. Nsc-758638
50. Tak-390mr
51. 0k5c5t2qpg
52. Mls000069705
53. 1261394-42-6
54. Lanzol-30
55. 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole
56. 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)sulfinyl)-1h-benzo[d]imidazole
57. 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylsulfinyl]-1h-benzimidazole
58. Ncgc00015615-02
59. Smr000058469
60. Lansoprazol [inn-spanish]
61. Lansoprazolum [inn-latin]
62. Cas-103577-45-3
63. Dsstox_cid_3200
64. Mls-0003247.0001
65. Prevacid Naprapac
66. 1h-benzimidazole, 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-
67. 1h-benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-
68. 2-({[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}sulfinyl)-1h-benzimidazole
69. 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-1h-benzimidazole
70. Dsstox_rid_76922
71. Dsstox_gsid_23200
72. Prevacid I.v.
73. Tak 390mr
74. Lansophed
75. Lanzol
76. Lanzul
77. 2-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methylsulfinyl)-1h-benzo[d]imidazole
78. 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]benzimidazole
79. Prevacid (tn)
80. 2-({3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl)methyl} Sulphinylbenzimidazole;2-({3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl)methyl} Sulphinylbenzimidazole
81. Hsdb 7204
82. Sr-01000000169
83. A 65006
84. Unii-0k5c5t2qpg
85. Brn 4333393
86. Lanfast
87. Lapraz
88. 2-{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl}-1h-benzimidazole
89. Abt-006
90. Lansoprazole,(s)
91. 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)sulfinyl)-1h-benzimidazole
92. Lansoprazole [usan:usp:inn:ban]
93. Mfcd00866873
94. Cg-4801
95. Spectrum_001580
96. Cpd000058469
97. Prevacid Delayed Release
98. Opera_id_1676
99. Prestwick0_001072
100. Prestwick1_001072
101. Prestwick2_001072
102. Prestwick3_001072
103. Spectrum2_000444
104. Spectrum3_000295
105. Spectrum4_000856
106. Spectrum5_001521
107. Dexlansoprazole-[13c6]
108. Lansoprazole [mi]
109. Lopac-l-8533
110. Lansoprazole [inn]
111. Lansoprazole [jan]
112. Chembl480
113. L 8533
114. Lansoprazole [hsdb]
115. Lansoprazole [usan]
116. Cid_3883
117. Lansoprazole [vandf]
118. Lopac0_000709
119. Schembl22365
120. Bspbio_001084
121. Bspbio_001830
122. Kbiogr_001491
123. Kbioss_002060
124. Lansoprazole [mart.]
125. Lansoprazole Impurity Standard
126. Mls000759405
127. Mls001074170
128. Mls001424235
129. (+/-)-lansoprazole
130. Bidd:gt0006
131. Divk1c_000920
132. Lansoprazole [usp-rs]
133. Lansoprazole [who-dd]
134. Spectrum1503926
135. Spbio_000488
136. Spbio_002992
137. Bpbio1_001194
138. Gtpl7208
139. Dtxsid4023200
140. Bdbm47032
141. Hms502n22
142. Kbio1_000920
143. Kbio2_002060
144. Kbio2_004628
145. Kbio2_007196
146. Kbio3_001330
147. Lansoprazole (jp17/usp/inn)
148. 2-[(r)-[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1h-benzimidazole
149. 2-[(s)-[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1h-benzimidazole
150. Levolansoprazole;(-)-lansoprazole
151. Ninds_000920
152. Hms1571g06
153. Hms1922m04
154. Hms2052f05
155. Hms2093m07
156. Hms2098g06
157. Hms2234b10
158. Hms3262m19
159. Hms3264e12
160. Hms3269d15
161. Hms3371e21
162. Hms3394f05
163. Hms3413j05
164. Hms3654j17
165. Hms3677j05
166. Hms3715g06
167. Lansoprazole [orange Book]
168. Pharmakon1600-01503926
169. Pharmakon1600-01504282
170. Lansoprazole [ep Monograph]
171. Act03358
172. Bcp22331
173. Bcp34129
174. Lansoprazole For Peak Identification
175. Lansoprazole [usp Monograph]
176. Tox21_110184
177. Tox21_301023
178. Tox21_500709
179. Bbl029072
180. Bdbm50070208
181. Ccg-39952
182. Nsc758638
183. Nsc758710
184. Prevpac Component Lansoprazole
185. S1354
186. Stk621169
187. Akos005554811
188. Tox21_110184_1
189. Ac-1233
190. Cs-1847
191. Db00448
192. Ks-1075
193. Lansoprazole, >=98% (tlc), Powder
194. Lp00709
195. Nc00411
196. Nsc 758638
197. Sb19127
198. Sdccgsbi-0050687.p004
199. 2-[({3-methyl-4-[(2,2,2-trifluoroethyl)oxy]pyridin-2-yl}methyl)sulfinyl]-1h-benzimidazole
200. 2-{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methanesulfinyl}-1h-1,3-benzodiazole
201. Idi1_000920
202. Lansoprazole Component Of Prevpac
203. Ncgc00015615-01
204. Ncgc00015615-03
205. Ncgc00015615-04
206. Ncgc00015615-05
207. Ncgc00015615-06
208. Ncgc00015615-07
209. Ncgc00015615-08
210. Ncgc00015615-09
211. Ncgc00015615-10
212. Ncgc00015615-11
213. Ncgc00015615-12
214. Ncgc00015615-14
215. Ncgc00015615-15
216. Ncgc00023826-03
217. Ncgc00023826-04
218. Ncgc00023826-05
219. Ncgc00023826-06
220. Ncgc00023826-07
221. Ncgc00254925-01
222. Ncgc00261394-01
223. Ncgc00381720-13
224. Bl166173
225. Hy-13662
226. Sbi-0050687.p003
227. Ab00052388
228. Eu-0100709
229. Ft-0610894
230. Ft-0670721
231. Ft-0670722
232. L0233
233. Sw197226-4
234. D00355
235. F20528
236. Ab00052388-17
237. Ab00052388_18
238. Ab00052388_19
239. 577l453
240. A800764
241. A921066
242. Q254296
243. J-007154
244. Sr-01000000169-2
245. Sr-01000000169-6
246. Sr-01000000169-9
247. Brd-a49172652-001-05-7
248. Brd-a49172652-001-13-1
249. F2173-0222
250. Lansoprazole, British Pharmacopoeia (bp) Reference Standard
251. Lansoprazole, European Pharmacopoeia (ep) Reference Standard
252. Lansoprazole, United States Pharmacopeia (usp) Reference Standard
253. (+)-2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-1h-benzimidazole
254. 2-({[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methane}sulfinyl)-1h-1,3-benzodiazole
255. 2-[[ [3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]-1h-benzimidazole
256. 2-[[[3-methyl-4-(2,2,2-trifluoro-ethoxy)-2-pyridinyl]methyl]-sulfinyl]1h-benzimidazole
257. 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]benzimidazole
258. 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]-1h-benzimidazole
259. 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylsulfinyl]-1h-benzimidazole
260. 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyrid-2-yl]methylsulfinyl]benzimidazole
261. 2-[[3-methyl-4-[2,2,2-tris(fluoranyl)ethoxy]pyridin-2-yl]methylsulfinyl]-1h-benzimidazole
262. 2-[3-methyl-4-(2,2,2-trifluoro-ethoxy)-pyridin-2-ylmethanesulfinyl]-1h-benzimidazole
263. 2-[3-methyl-4-(2,2,2-trifluoro-ethoxy)-pyridin-2-ylmethanesulfinyl]-benzimidazole
264. 2-[3-methyl-4-(2,2,2-trifluoro-ethoxy)pyridin-2-ylmethanesulfinyl]-benzimidazole
265. 2-[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl-methanesulfinyl]-1h-benzimidazole
266. 2-[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-ylmethanesulfinyl]-1h-benzimidazole
267. Lansoprazole For Peak Identification, European Pharmacopoeia (ep) Reference Standard
268. 1h-benzimidazole, 2-[(s)-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-
269. 2-[[[3-methyl-4-(2,2,2-trifluoro-ethoxy)-pyridin-2-yl]methyl]sulfinyl]-1h-benzo[d]imidazole
| Molecular Weight | 369.4 g/mol |
|---|---|
| Molecular Formula | C16H14F3N3O2S |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 369.07588236 g/mol |
| Monoisotopic Mass | 369.07588236 g/mol |
| Topological Polar Surface Area | 87.1 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Lansoprazole |
| PubMed Health | Lansoprazole (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in lansoprazole delayed-release capsules USP is lansoprazole, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Lansop... |
| Active Ingredient | Lansoprazole |
| Dosage Form | Capsule, delayed rel pellets |
| Route | Oral |
| Strength | 30mg; 15mg |
| Market Status | Over the Counter; Prescription |
| Company | Wockhardt; Mylan Pharms; Krka Tovarna Zdravil; Natco Pharma; Sandoz; Sun Pharma Global; Perrigo R And D; Teva Pharms; Zydus Hlthcare; Dr Reddys Labs; Wockhardt Usa |
| 2 of 4 | |
|---|---|
| Drug Name | Prevacid |
| PubMed Health | Naproxen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | The active ingredient in PREVACID Delayed-Release Capsules and PREVACID SoluTab Delayed-Release Orally Disintegrating Tablets is lansoprazole, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimida... |
| Active Ingredient | Lansoprazole |
| Dosage Form | Capsule, delayed rel pellets; Tablet, delayed release, orally disintegrating |
| Route | Oral |
| Strength | 30mg; 15mg |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 3 of 4 | |
|---|---|
| Drug Name | Lansoprazole |
| PubMed Health | Lansoprazole (By mouth) |
| Drug Classes | Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in lansoprazole delayed-release capsules USP is lansoprazole, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Lansop... |
| Active Ingredient | Lansoprazole |
| Dosage Form | Capsule, delayed rel pellets |
| Route | Oral |
| Strength | 30mg; 15mg |
| Market Status | Over the Counter; Prescription |
| Company | Wockhardt; Mylan Pharms; Krka Tovarna Zdravil; Natco Pharma; Sandoz; Sun Pharma Global; Perrigo R And D; Teva Pharms; Zydus Hlthcare; Dr Reddys Labs; Wockhardt Usa |
| 4 of 4 | |
|---|---|
| Drug Name | Prevacid |
| PubMed Health | Naproxen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | The active ingredient in PREVACID Delayed-Release Capsules and PREVACID SoluTab Delayed-Release Orally Disintegrating Tablets is lansoprazole, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimida... |
| Active Ingredient | Lansoprazole |
| Dosage Form | Capsule, delayed rel pellets; Tablet, delayed release, orally disintegrating |
| Route | Oral |
| Strength | 30mg; 15mg |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
Antiulcerative
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 961
Lansoprazole is indicated for the short-term treatment of heartburn and other symptoms associated with gastroesophageal reflux disease (GERD). Lansoprazole is indicated for the short-term (up to 8 weeks) treatment for symptom relief and healing of all grades of erosive esophagitis (associated with GERD). Lansoprazole may be indicated for an additional 8 weeks of treatment of patients in whom healing has not occurred. If erosive esophagitis recurs, an additional course of lansoprazole treatment may be considered. Lansoprazole also is indicated to maintain healing of erosive esophagitis. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1724
Lansoprazole is indicated for short-term (up to 8 weeks) treatment in patients with active benign gastric ulcer. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1724
Lansoprazole is indicated for short-term (up to 4 weeks) treatment for symptom relief and healing in patients with active duodenal ulcer. Lansoprazole also is indicated to maintain healing of duodenal ulcers. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1724
For more Therapeutic Uses (Complete) data for LANSOPRAZOLE (6 total), please visit the HSDB record page.
Worldwide, over 10,000 patients have been treated with lansoprazole in Phase 2-3 trials involving various dosages and durations of treatment. The adverse reaction profiles for prevacid delayed-release capsules and prevacid for delayed-release oral suspension are similar. In general, lansoprazole treatment has been well-tolerated in both short-term and long-term trials. ... The most commonly reported possibly or probably treatment-related adverse event during maintenance therapy was diarrhea.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 3211
An 85-year-old white man presented with an upper gastrointestinal hemorrhage from a gastric ulcer. His platelet count was normal on admission. He was started on oral lansoprazole 60 mg twice daily and, on hospital day 2, his platelet count decreased to 102 x 10(3)/mm(3); on hospital day 3, the platelet count was 36 x 10(3)/mm(3). Lansoprazole was discontinued, and the platelet count returned to normal. He has not had any further episodes of thrombocytopenia to date. After exclusion of other causes, the onset of thrombocytopenia after administration of lansoprazole, the resolution of the adverse reaction after discontinuation of the drug, and the fact that no other medicines were introduced during this time frame lead us to believe that this was most likely an idiosyncratic thrombocytopenic response to lansoprazole. To date, this is the first reported case of what appears to be isolated thrombocytopenia associated with lansoprazole.
Zlabek JA, Anderson CG: Ann Pharmacother 36 (5): 809-11 (2002)
Studies in elderly patients indicate that the clearance of lansoprazole is decreased in the elderly, resulting in a 50 to 100% increase in the elimination half-life. Because the mean half-life in the elderly remains between 1.9 and 2.9 hours, repeated once-daily dosing does not result in accumulation of lansoprazole. However, subsequent doses higher than 30 mg a day should not be administered unless additional gastric acid suppression is necessary.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
Diarrhea is one of the most frequently reported adverse events during proton pump inhibitor use in any setting. Because of the limited available information, this study was set up with the aim of assessing the incidence and characteristics of diarrhea and to investigate possible associated co-factors in proton pump inhibitor users in daily practice. Data were used from a prospective, observational study in which 10,008 lansprazole users were followed over time (1994-1998). The study was designed according to the SAMM guidelines. A nested case-control design was used to compare proton pump inhibitor users reporting diarrhea with those reporting no diarrhea. The frequency of diarrhea was 3.7% and the incidence density 10.7 per 1000 patients months of proton pump inhibitor use. The diarrhea was most commonly loose and occurred on average 4.4 times per day. The analysis of co-factors revealed that patients with concomitant use of oral antibiotics and patients reporting neurological and/or dermatological adverse events, were at risk of developing diarrhea during proton pomp inhibitor use.
PMID:12512247 Claessens AA et al; Pharmacoepidemiol Drug Saf 11 (8): 703-8 (2002)
For more Drug Warnings (Complete) data for LANSOPRAZOLE (8 total), please visit the HSDB record page.
Lansoprazole is used to reduce gastric acid secretion and is approved for short term treatment of active gastric ulcers, active duodenal ulcers, erosive reflux oesophagitis, symptomatic gastroesophageal reflux disease, and non-steroidal anti-inflammatory drug (NSAID) induced gastric and duodenal ulcers. It may be used in the maintenance and healing of several gastric conditions including duodenal ulcers, NSAID related gastric ulcers, and erosive esophagitis. Lansoprazole prevents recurrence of gastric ulcers in patients who have a documented history of gastric ulcers who also use NSAIDs chronically. Predictably, it is also useful in the management of hypersecretory conditions including Zollinger-Ellison syndrome. Lansoprazole is effective at eradicating H. pylori when used in conjunction with amoxicillin and clarithromycin (triple therapy) or with amoxicillin alone (dual therapy).
FDA Label
Lansoprazole decreases gastric acid secretion by targeting H+,K+-ATPase, which is the enzyme that catalyzes the final step in the acid secretion pathway in parietal cells. Conveniently, lansoprazole administered any time of day is able to inhibit both daytime and nocturnal acid secretion. The result is that lansoprazole is effective at healing duodenal ulcers, reduces ulcer-related pain, and offers relief from symptoms of heartburn Lansoprazole also reduces pepsin secretion, making it a useful treatment option for hypersecretory conditions such as Zollinger-Ellison syndrome.
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
A02BC03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BC - Proton pump inhibitors
A02BC03 - Lansoprazole
Absorption
The oral bioavailability of lansoprazole is reported to be 80-90% and the peak plasma concentration(Cmax) is achieved about 1.7 hours after oral dosing. Food reduces the absorption of lansoprazole (both Cmax and AUC are reduced by 50-70%); therefore, patients should be instructed to take lansoprazole before meals.
Route of Elimination
A reported 14-23% of a lansoprazole is eliminated in the urine with this percentage range including both conjugated and unconjugated hydroxylated metabolites.
Volume of Distribution
The apparent volume of distribution of lansoprazole is 0.4 L/kg.
Clearance
The reported clearance of lansoprazole is 400-650 mL/min.
Very high (around 97%) /protein binding/; protein binding remains constant over the concentration range of 0.05 to 5 ug/mL. In patient with renal function impairment, protein binding may be decreased by 1 to 1.5%.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
Distributed in tissue, particularly gastric parietal cells. Apparent oral volume of distribution following administration of 30 mg of lansoprazole is about 0.5 L/kg.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
Since lansoprazole is acid-labile, it is administered as a capsule containing enter-coated granules to prevent gastric decomposition and to increase bioavailability. Once lansoprazole has left the stomach, absorption is rapid and relatively complete, with absolute bioavailability over 80%. Bioavailability may be decreased if lansoprazole is administered within 30 minutes of food intake as compared to that of a fasting state. Absorption may be delayed in patients with hepatic cirrhosis.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
Elimination: Renal: Approximately 14 to 25% of a dose of lansoprazole is excreted in the urine, as conjugated and unconjugated hydroxylated metabolites. Less than 1% of unchanged lansoprazole is detectable in the urine. Biliary/fecal: Approximately two-thirds of a dose of lansoprazole is detected as metabolites in the feces. In dialysis: Lansoprazole and its metabolites are not significantly dialyzed; no appreciable fraction is removed by hemodialysis. Note: Elimination is prolonged in healthy elderly subjects, in adult and elderly patient with mild renal impairment, and in patients with severe liver disease.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
For more Absorption, Distribution and Excretion (Complete) data for LANSOPRAZOLE (6 total), please visit the HSDB record page.
Lansoprazole is predominantly metabolized in the liver by CYP3A4 and CYP2C19. The resulting major metabolites are 5-hydroxy lansoprazole and the sulfone derivative of lansoprazole.
Lansoprazole is extensively metabolized in the liver to two main excretory metabolites that are inactive. In the acidic environment of the gastric parietal cell, lansoprazole is converted to two active compounds that inhibit acid secretion by (H+,K+)-ATPase within the parietal cell canaliculus, but that are not present in the systemic circulation.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
One source reports the half life of lansoprazole to be 0.9 - 1.6 hours, while another source cites 0.9 - 2.1 hours. The general consensus is that lansoprazole has a short half life and is approximately 2 hours or less. These numbers may be misleading since it suggests that lansoprazole has a short duration of action when in practice, lansoprazole can effectively inhibit acid secretion for ~24 hours due to it's mechanism of action.
Elimination: Normal renal function: Approximately 1.5 hours. Renal function impairment: Shortened elimination half-life. Elderly patients: 1.9 to 2.9 hours. Hepatic function impairment: 3.2 to 7.2 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1725
As a PPI, lansoprazole is a prodrug and requires protonation via an acidic environment to become activated. Once protonated, lansoprazole is able to react with cysteine residues, specifically Cys813 and Cys321, on parietal H+,K+-ATPase resulting in stable disulfides. PPI's in general are able to provide prolonged inhibition of acid secretion due to their ability to bind covalently to their targets.
Lansoprazole is a selective and irreversible proton pump inhibitor. In the acidic environment of the gastric parietal cell, lansoprazole is converted to active sulphenamide derivatives that bind to the sulfhydryl group of (H+, K+)-adenosine triphosphatase ((H+,K+)-ATPase), also known as the proton pump (H+,K+)-ATPase catalyzes the final step in the gastric acid secretion pathway. Lansoprazole's inhibition of (H+,K+)-ATPase results in inhibition of both centrally and peripherally mediated gastric acid secretion. The inhibitory effect is dose-related. Lansoprazole inhibits both basal and stimulated gastric acid secretion regardless of the stimulus.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1724

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
96
PharmaCompass offers a list of Lansoprazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lansoprazole manufacturer or Lansoprazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lansoprazole manufacturer or Lansoprazole supplier.
PharmaCompass also assists you with knowing the Lansoprazole API Price utilized in the formulation of products. Lansoprazole API Price is not always fixed or binding as the Lansoprazole Price is obtained through a variety of data sources. The Lansoprazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lansoprazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lansoprazole, including repackagers and relabelers. The FDA regulates Lansoprazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lansoprazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lansoprazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lansoprazole supplier is an individual or a company that provides Lansoprazole active pharmaceutical ingredient (API) or Lansoprazole finished formulations upon request. The Lansoprazole suppliers may include Lansoprazole API manufacturers, exporters, distributors and traders.
click here to find a list of Lansoprazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lansoprazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Lansoprazole active pharmaceutical ingredient (API) in detail. Different forms of Lansoprazole DMFs exist exist since differing nations have different regulations, such as Lansoprazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lansoprazole DMF submitted to regulatory agencies in the US is known as a USDMF. Lansoprazole USDMF includes data on Lansoprazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lansoprazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lansoprazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lansoprazole Drug Master File in Japan (Lansoprazole JDMF) empowers Lansoprazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lansoprazole JDMF during the approval evaluation for pharmaceutical products. At the time of Lansoprazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lansoprazole suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lansoprazole Drug Master File in Korea (Lansoprazole KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lansoprazole. The MFDS reviews the Lansoprazole KDMF as part of the drug registration process and uses the information provided in the Lansoprazole KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lansoprazole KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lansoprazole API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lansoprazole suppliers with KDMF on PharmaCompass.
A Lansoprazole CEP of the European Pharmacopoeia monograph is often referred to as a Lansoprazole Certificate of Suitability (COS). The purpose of a Lansoprazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lansoprazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lansoprazole to their clients by showing that a Lansoprazole CEP has been issued for it. The manufacturer submits a Lansoprazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lansoprazole CEP holder for the record. Additionally, the data presented in the Lansoprazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lansoprazole DMF.
A Lansoprazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lansoprazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lansoprazole suppliers with CEP (COS) on PharmaCompass.
A Lansoprazole written confirmation (Lansoprazole WC) is an official document issued by a regulatory agency to a Lansoprazole manufacturer, verifying that the manufacturing facility of a Lansoprazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lansoprazole APIs or Lansoprazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Lansoprazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Lansoprazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lansoprazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lansoprazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lansoprazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lansoprazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lansoprazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lansoprazole suppliers with NDC on PharmaCompass.
Lansoprazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lansoprazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lansoprazole GMP manufacturer or Lansoprazole GMP API supplier for your needs.
A Lansoprazole CoA (Certificate of Analysis) is a formal document that attests to Lansoprazole's compliance with Lansoprazole specifications and serves as a tool for batch-level quality control.
Lansoprazole CoA mostly includes findings from lab analyses of a specific batch. For each Lansoprazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lansoprazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Lansoprazole EP), Lansoprazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lansoprazole USP).
