
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
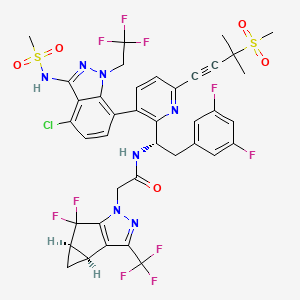

1. Gs-ca1
1. Gs-6207
2. 2189684-44-2
3. Gs-hiv
4. Gs-ca2
5. Gs-ca-2
6. Lenacapavir [usan]
7. Gs-ca1
8. A9a0o6fb4h
9. Gs-714207
10. Gs6207
11. N-[(1s)-1-[3-[4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)indazol-7-yl]-6-(3-methyl-3-methylsulfonylbut-1-ynyl)pyridin-2-yl]-2-(3,5-difluorophenyl)ethyl]-2-[(2s,4r)-5,5-difluoro-9-(trifluoromethyl)-7,8-diazatricyclo[4.3.0.02,4]nona-1(6),8-dien-7-yl]acetamide
12. N-((s)-1-(3-(4-chloro-3-(methylsulfonamido)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)but-1-yn-1-yl)pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl)-2-((3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl)acetamide
13. N-[(1s)-1-(3-{4-chloro-3-[(methylsulfonyl)amino]-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl}-6-[3-methyl-3-(methylsulfonyl)but-1-yn-1-yl]pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl]-2-[(3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl]acetamide
14. Qng
15. Lenacapavir [inn]
16. Unii-a9a0o6fb4h
17. Lenacapavir [who-dd]
18. Chembl4594438
19. Schembl19875642
20. Gtpl11446
21. Ex-a5518
22. Who 11108
23. At20076
24. Hy-111964
25. Cs-0094695
26. 1h-cyclopropa(3,4)cyclopenta(1,2-c)pyrazole, N-((1s)-1-(3-(4-chloro-3-((methylsulfonyl)amino)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)-1-butyn-1-yl)-2-pyridinyl)-2-(3,5-difluorophenyl)ethyl)-5,5-difluoro-3b,4,4a,5-tetrahydro-3-(trifluoromethyl)-, (3bs,4ar)-
27. N-((1s)-1-(3-(4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-(methanesulfonyl)-3-methylbut-1-yn-1-yl)pyridin-2-yl)-2-(3,5- Difluorophenyl)ethyl)-2-((3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa(3,4)cyclopenta(1,2-c)pyrazol-1-yl)acetamide
28. N-[(1s)-1-[3-[4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)indazol-7-yl]-6-(3-methyl-3-methylsulfonyl-but-1-ynyl)-2-pyridyl]-2-(3,5-difluorophenyl)ethyl]-2-[difluoro(trifluoromethyl)[?]yl]acetamide
| Molecular Weight | 968.3 g/mol |
|---|---|
| Molecular Formula | C39H32ClF10N7O5S2 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 13 |
| Exact Mass | 967.1435188 g/mol |
| Monoisotopic Mass | 967.1435188 g/mol |
| Topological Polar Surface Area | 175 Ų |
| Heavy Atom Count | 64 |
| Formal Charge | 0 |
| Complexity | 2040 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of human immunodeficiency virus (HIV-1) infection
* Sunlenca injection: , in combination with other antiretroviral(s), is indicated for the treatment of adults with multidrug resistant HIV 1 infection for whom it is otherwise not possible to construct a suppressive anti viral regimen (see sections 4. 2 and 5. 1).
* Sunlenca tablet: , in combination with other antiretroviral(s), is indicated for the treatment of adults with multidrug resistant HIV 1 infection for whom it is otherwise not possible to construct a suppressive anti viral regimen, for oral loading prior to administration of long-acting lenacapavir injection (see sections 4. 2 and 5. 1).
J05AX
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX31 - Lenacapavir
NDC Package Code : 54014-6902
Start Marketing Date : 2022-12-22
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 66721-830
Start Marketing Date : 2022-08-17
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (20kg/20kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Viatris
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Viatris
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Gilead Partners with Six Generics For Lenacapavir Access in HIV Prevention
Details : The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Hetero
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Hetero
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Hetero Partners with Gilead To Expand HIV Drug Lenacapavir Access Globally
Details : The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Dr. Reddy\'s Laboratories
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Dr. Reddy\'s Laboratories
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Dr. Reddy’s Partners with Gilead to Manufacture Lenacapavir in India and Beyond
Details : The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Emcure Pharmaceuticals
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Emcure Pharmaceuticals
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Gilead Partners with Six Generic Makers to Expand HIV Prevention Access Globally
Details : The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Eva Pharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Eva Pharma
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Gilead Partners with Six Generic Makers to Expand HIV Prevention Access Globally
Details : The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Ferozsons Laboratories
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Ferozsons Laboratories
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Gilead Partners with Six Generic Makers to Expand HIV Prevention Access Globally
Details : The agreement aims to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries for HIV prevention.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MK-8591 (islatravir) is a non-nucleoside reverse transcriptase translocation inhibitor, which is being investigated in phase 2 for HIV-1 infection, in combination with lenacapavir.
Lead Product(s): Islatravir,Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: MK-8591
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Merck & Co
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Islatravir,Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : Not Applicable
Deal Type : Not Applicable
Gilead and Merck Announce Phase 2 Data for Islatravir and Lenacapavir Combination Regimen
Details : MK-8591 (islatravir) is a non-nucleoside reverse transcriptase translocation inhibitor, which is being investigated in phase 2 for HIV-1 infection, in combination with lenacapavir.
Brand Name : MK-8591
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GS-9883 (bictegravir), an HIV type 1 integrase inhibitor in combination with GS-6207 (lenacapavir), a structural capsid protein inhibitor is being evaluated for the treatment of HIV-1-infection.
Lead Product(s): Bictegravir,Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: GS-9883
Study Phase: Phase II/ Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bictegravir,Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Gilead’s Innovative HIV Research Pipeline Aims to Address Unmet Needs and Advance Health
Details : GS-9883 (bictegravir), an HIV type 1 integrase inhibitor in combination with GS-6207 (lenacapavir), a structural capsid protein inhibitor is being evaluated for the treatment of HIV-1-infection.
Brand Name : GS-9883
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 18, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 18, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Lead Product(s): Lenacapavir,Teropavimab,Zinlirvimab
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 22, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir,Teropavimab,Zinlirvimab
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 22, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]2-((3bS,4aR)-5,5-Difluoro-3-(trifluoromethyl)-3b,4...
CAS Number : 1620056-83-8
End Use API : Lenacapavir
About The Company : Headquartered in Fengxian District, Shanghai Minbiotch Co., Ltd. is a company specializing in the R&D and production of advanced pharmaceutical intermediates...
3-Methyl-3-(methylsulfonyl)but-1-yne
CAS Number : 2109226-54-0
End Use API : Lenacapavir
About The Company : Headquartered in Fengxian District, Shanghai Minbiotch Co., Ltd. is a company specializing in the R&D and production of advanced pharmaceutical intermediates...
tert-butylN-[(1S)-1-(3,6-dibromopyridin-2-yl)-2-(3...
CAS Number : 1620056-47-4
End Use API : Lenacapavir
About The Company : Headquartered in Fengxian District, Shanghai Minbiotch Co., Ltd. is a company specializing in the R&D and production of advanced pharmaceutical intermediates...
Carbamic acid, N-[(1S)-1-[3-bromo-6-[3-methyl-3-(...
CAS Number : 2189684-54-4
End Use API : Lenacapavir
About The Company : Headquartered in Fengxian District, Shanghai Minbiotch Co., Ltd. is a company specializing in the R&D and production of advanced pharmaceutical intermediates...
4-Chloro-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan...
CAS Number : 2189684-53-3
End Use API : Lenacapavir
About The Company : Headquartered in Fengxian District, Shanghai Minbiotch Co., Ltd. is a company specializing in the R&D and production of advanced pharmaceutical intermediates...
2-((3bS,4aR)-5,5-difluoro-3- (trifluoromethyl)-3b,...
CAS Number : 1620056-83-8
End Use API : Lenacapavir
About The Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & ope...
CAS Number : 91-00-9
End Use API : Lenacapavir
About The Company : SCI Pharmtech Inc. offers APIs, advanced intermediates, and custom products, focusing on quick development and cost-effective production. Our research labs, pil...
CAS Number : 91-00-9
End Use API : Lenacapavir
About The Company : SCI Pharmtech Inc. offers APIs, advanced intermediates, and custom products, focusing on quick development and cost-effective production. Our research labs, pil...
4-Chloro-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan...
CAS Number : 2189684-53-3
End Use API : Lenacapavir
About The Company : Saptagir Laboratories Private incorporated in 2016, is a manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) and Intermediates for a wide rang...

Tert-butyl (S)-[1-(3-bromo-6-[3-methyl-3-(methyl s...
CAS Number : 2189684-54-4
End Use API : Lenacapavir
About The Company : Saptagir Laboratories Private incorporated in 2016, is a manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) and Intermediates for a wide rang...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : SUNLENCA
Dosage Form : SOLUTION;SUBCUTANEOUS
Dosage Strength : EQ 463.5MG BASE/1.5ML (EQ 309MG BASE/ML)
Packaging :
Approval Date : 2022-12-22
Application Number : 215973
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : SUNLENCA
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 300MG BASE
Packaging :
Approval Date : 2022-12-22
Application Number : 215974
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : SUNLENCA
Dosage Form : SOLUTION;SUBCUTANEOUS
Dosage Strength : EQ 463.5MG BASE/1.5ML (EQ 309MG BASE/ML)
Approval Date : 2022-12-22
Application Number : 215973
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : SUNLENCA
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 300MG BASE
Approval Date : 2022-12-22
Application Number : 215974
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]https://www.pharmacompass.com/radio-compass-blog/fda-s-drug-approvals-drop-26-due-to-covid-okays-three-costly-gene-therapies-in-h2
Patents & EXCLUSIVITIES
Patent Expiration Date : 2034-02-28
US Patent Number : 9951043
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215974
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2034-02-28

Patent Expiration Date : 2037-08-17
US Patent Number : 10654827
Drug Substance Claim :
Drug Product Claim :
Application Number : 215974
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2038-08-16
US Patent Number : 11267799
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 215974
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2038-08-16

Patent Expiration Date : 2037-08-17
US Patent Number : 10071985
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215973
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2041-06-04
US Patent Number : 11944611
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215974
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2041-06-04

Patent Expiration Date : 2037-08-17
US Patent Number : 10071985
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215974
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2041-06-04
US Patent Number : 11944611
Drug Substance Claim :
Drug Product Claim :
Application Number : 215973
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2041-06-04

Patent Expiration Date : 2038-08-16
US Patent Number : 11267799
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 215973
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2038-08-16

Patent Expiration Date : 2037-08-17
US Patent Number : 10654827
Drug Substance Claim :
Drug Product Claim :
Application Number : 215973
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2034-02-28
US Patent Number : 9951043
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215973
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2034-02-28

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2027-12-22
Application Number : 215974
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2027-12-22
Application Number : 215973
Product Number : 1
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?