

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
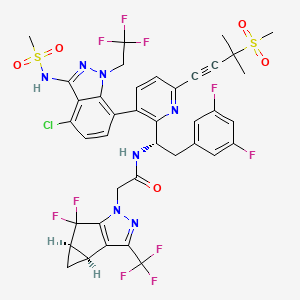

1. Gs-ca1
1. Gs-6207
2. 2189684-44-2
3. Gs-hiv
4. Gs-ca2
5. Gs-ca-2
6. Lenacapavir [usan]
7. Gs-ca1
8. A9a0o6fb4h
9. Gs-714207
10. Gs6207
11. N-[(1s)-1-[3-[4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)indazol-7-yl]-6-(3-methyl-3-methylsulfonylbut-1-ynyl)pyridin-2-yl]-2-(3,5-difluorophenyl)ethyl]-2-[(2s,4r)-5,5-difluoro-9-(trifluoromethyl)-7,8-diazatricyclo[4.3.0.02,4]nona-1(6),8-dien-7-yl]acetamide
12. N-((s)-1-(3-(4-chloro-3-(methylsulfonamido)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)but-1-yn-1-yl)pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl)-2-((3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl)acetamide
13. N-[(1s)-1-(3-{4-chloro-3-[(methylsulfonyl)amino]-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl}-6-[3-methyl-3-(methylsulfonyl)but-1-yn-1-yl]pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl]-2-[(3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl]acetamide
14. Qng
15. Lenacapavir [inn]
16. Unii-a9a0o6fb4h
17. Lenacapavir [who-dd]
18. Chembl4594438
19. Schembl19875642
20. Gtpl11446
21. Ex-a5518
22. Who 11108
23. At20076
24. Hy-111964
25. Cs-0094695
26. 1h-cyclopropa(3,4)cyclopenta(1,2-c)pyrazole, N-((1s)-1-(3-(4-chloro-3-((methylsulfonyl)amino)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)-1-butyn-1-yl)-2-pyridinyl)-2-(3,5-difluorophenyl)ethyl)-5,5-difluoro-3b,4,4a,5-tetrahydro-3-(trifluoromethyl)-, (3bs,4ar)-
27. N-((1s)-1-(3-(4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-(methanesulfonyl)-3-methylbut-1-yn-1-yl)pyridin-2-yl)-2-(3,5- Difluorophenyl)ethyl)-2-((3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa(3,4)cyclopenta(1,2-c)pyrazol-1-yl)acetamide
28. N-[(1s)-1-[3-[4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)indazol-7-yl]-6-(3-methyl-3-methylsulfonyl-but-1-ynyl)-2-pyridyl]-2-(3,5-difluorophenyl)ethyl]-2-[difluoro(trifluoromethyl)[?]yl]acetamide
| Molecular Weight | 968.3 g/mol |
|---|---|
| Molecular Formula | C39H32ClF10N7O5S2 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 13 |
| Exact Mass | 967.1435188 g/mol |
| Monoisotopic Mass | 967.1435188 g/mol |
| Topological Polar Surface Area | 175 Ų |
| Heavy Atom Count | 64 |
| Formal Charge | 0 |
| Complexity | 2040 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of human immunodeficiency virus (HIV-1) infection
* Sunlenca injection: , in combination with other antiretroviral(s), is indicated for the treatment of adults with multidrug resistant HIV 1 infection for whom it is otherwise not possible to construct a suppressive anti viral regimen (see sections 4. 2 and 5. 1).
* Sunlenca tablet: , in combination with other antiretroviral(s), is indicated for the treatment of adults with multidrug resistant HIV 1 infection for whom it is otherwise not possible to construct a suppressive anti viral regimen, for oral loading prior to administration of long-acting lenacapavir injection (see sections 4. 2 and 5. 1).
J05AX
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX31 - Lenacapavir
NDC Package Code : 54014-6902
Start Marketing Date : 2022-12-22
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 66721-830
Start Marketing Date : 2022-08-17
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (20kg/20kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MK-8591 (islatravir) is a non-nucleoside reverse transcriptase translocation inhibitor, which is being investigated in phase 2 for HIV-1 infection, in combination with lenacapavir.
Lead Product(s): Islatravir,Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: MK-8591
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Merck & Co
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Islatravir,Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MK-8591 (islatravir) is a non-nucleoside reverse transcriptase translocation inhibitor, which is being investigated in phase 2 for HIV-1 infection, in combination with lenacapavir.
Brand Name : MK-8591
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 06, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GS-9883 (bictegravir), an HIV type 1 integrase inhibitor in combination with GS-6207 (lenacapavir), a structural capsid protein inhibitor is being evaluated for the treatment of HIV-1-infection.
Lead Product(s): Bictegravir,Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: GS-9883
Study Phase: Phase II/ Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bictegravir,Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Gilead’s Innovative HIV Treatment Research Pipeline Aims to Address Unmet Needs and Advance Publ...
Details : GS-9883 (bictegravir), an HIV type 1 integrase inhibitor in combination with GS-6207 (lenacapavir), a structural capsid protein inhibitor is being evaluated for the treatment of HIV-1-infection.
Brand Name : GS-9883
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 18, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 18, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Lead Product(s): Lenacapavir,Teropavimab,Zinlirvimab
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 22, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir,Teropavimab,Zinlirvimab
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Sunlenca (lenacapavir) is a selective long-acting inhibitor of HIV-1 capsid function which inhibits HIV-1 replication by interfering with multiple essential steps of the viral lifecycle.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 22, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca is the first of a new class of drugs called capsid inhibitors to be FDA-approved for treating HIV-1. Sunlenca works by blocking the HIV-1 virus’ protein shell (the capsid), thereby interfering with multiple essential steps of the viral lifecycle.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
FDA Approves New HIV Drug for Adults with Limited Treatment Options
Details : Sunlenca is the first of a new class of drugs called capsid inhibitors to be FDA-approved for treating HIV-1. Sunlenca works by blocking the HIV-1 virus’ protein shell (the capsid), thereby interfering with multiple essential steps of the viral lifecyc...
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca (lenacapavir) is a first-in-class, long-acting HIV capsid inhibitor approved in the United States, the United Kingdom, Canada and the European Union, for the treatment of HIV infection, in combination with other antiretroviral.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Sunlenca® (lenacapavir) Receives FDA Approval as a First-in-Class, Twice-Yearly Treatment Option ...
Details : Sunlenca (lenacapavir) is a first-in-class, long-acting HIV capsid inhibitor approved in the United States, the United Kingdom, Canada and the European Union, for the treatment of HIV infection, in combination with other antiretroviral.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sunlenca (lenacapavir), is first-in-class capsid inhibitor with multi-stage mechanism of action, offering a new, every six-month treatment option for people with HIV whose virus no longer effectively responds to their current therapy.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Gilead Announces First Global Regulatory Approval of Sunlenca® (lenacapavir), the Only Twice-Year...
Details : Sunlenca (lenacapavir), is first-in-class capsid inhibitor with multi-stage mechanism of action, offering a new, every six-month treatment option for people with HIV whose virus no longer effectively responds to their current therapy.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The NDA resubmission contains comprehensive CMC data to support the compatibility of GS-6207 (lenacapavir), first-in-class, investigational long-acting HIV-1 capsid inhibitor with an alternative vial type made from aluminosilicate glass.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase II/ Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 27, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : The NDA resubmission contains comprehensive CMC data to support the compatibility of GS-6207 (lenacapavir), first-in-class, investigational long-acting HIV-1 capsid inhibitor with an alternative vial type made from aluminosilicate glass.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 27, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Recommendation is based on week 26 Data from the CAPELLA Trial showing twice-yearly GS-6207 (lenacapavir) achieved high rates of virologic suppression in heavily treatment-experienced people with HIV.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sunlenca
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Recommendation is based on week 26 Data from the CAPELLA Trial showing twice-yearly GS-6207 (lenacapavir) achieved high rates of virologic suppression in heavily treatment-experienced people with HIV.
Brand Name : Sunlenca
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GS-6207 (Lenacapavir) is Gilead’s potential first-in-class, investigational long-acting HIV-1 capsid inhibitor in development for the treatment and prevention of HIV-1 infection. Lenacapavir was generally well tolerated in CAPELLA, with no serious adverse events.
Lead Product(s): Lenacapavir
Therapeutic Area: Infections and Infectious Diseases Brand Name: GS-6207
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 16, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lenacapavir
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
FDA Lifts Clinical Hold on Investigational Lenacapavir for the Treatment and Prevention of HIV
Details : GS-6207 (Lenacapavir) is Gilead’s potential first-in-class, investigational long-acting HIV-1 capsid inhibitor in development for the treatment and prevention of HIV-1 infection. Lenacapavir was generally well tolerated in CAPELLA, with no serious adve...
Brand Name : GS-6207
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 16, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]2-((3bS,4aR)-5,5-difluoro-3- (trifluoromethyl)-3b,...
CAS Number : 1620056-83-8
End Use API : Lenacapavir
About The Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & ope...
CAS Number : 91-00-9
End Use API : Lenacapavir
About The Company : SCI Pharmtech Inc. offers APIs, advanced intermediates, and custom products, focusing on quick development and cost-effective production. Our research labs, pil...
CAS Number : 91-00-9
End Use API : Lenacapavir
About The Company : SCI Pharmtech Inc. offers APIs, advanced intermediates, and custom products, focusing on quick development and cost-effective production. Our research labs, pil...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
Patent Expiration Date : 2041-06-04
US Patent Number : 11944611
Drug Substance Claim :
Drug Product Claim :
Application Number : 215973
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2041-06-04

Patent Expiration Date : 2037-08-17
US Patent Number : 10654827
Drug Substance Claim :
Drug Product Claim :
Application Number : 215973
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2034-02-28
US Patent Number : 9951043
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215973
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2034-02-28

Patent Expiration Date : 2034-02-28
US Patent Number : 9951043
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215974
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2034-02-28

Patent Expiration Date : 2037-08-17
US Patent Number : 10654827
Drug Substance Claim :
Drug Product Claim :
Application Number : 215974
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2038-08-16
US Patent Number : 11267799
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 215974
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2038-08-16

Patent Expiration Date : 2037-08-17
US Patent Number : 10071985
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215973
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2037-08-17
US Patent Number : 10071985
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215974
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2037-08-17

Patent Expiration Date : 2041-06-04
US Patent Number : 11944611
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 215974
Patent Use Code : U-3507
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2041-06-04

Patent Expiration Date : 2038-08-16
US Patent Number : 11267799
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 215973
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2038-08-16

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2027-12-22
Application Number : 215974
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2027-12-22
Application Number : 215973
Product Number : 1
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
