
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
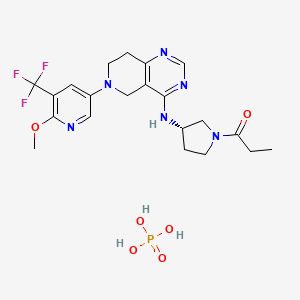

1. 1354691-97-6
2. Leniolisib Monophosphate
3. Joenja
4. Cdz173-az
5. 3700m1h39q
6. Cdz-173-az
7. Unii-3700m1h39q
8. Leniolisib (phosphate)
9. 1-propanone, 1-((3s)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-, Phosphate (1:1)
10. Cdz-173
11. 1-[(3s)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5h-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one;phosphoric Acid
12. 1-((3s)-3-((6-(6-methoxy-5-(trifluoromethyl)pyrimidin-3-yl)-5,6,7,8-tetrahydropyrido(4,3-d)pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one Phosphate
13. 1-[(3s)-3-({6-[6-methoxy-5-(trifluoromethyl)pyrimidin-3-yl]-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl}amino)pyrrolidin-1-yl]propan-1-one Phosphate
14. Joenja (tn)
15. Leniolisib Phosphate [who-dd]
16. Chembl3989909
17. Schembl15789519
18. Chebi:229650
19. Leniolisib Phosphate (jan/usan)
20. Xxedegoaysgnps-zownyotgsa-n
21. Leniolisib Phosphate [usan]
22. Hy-17635a
23. Akos040758719
24. Da-74950
25. Cs-0069337
26. D11159
27. Q27256604
28. (s)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one Phosphate
29. 1-[(3s)-3-({6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl}amino)pyrrolidin-1-yl]propan-1-one Phosphate
30. Phosphoric Acid--1-[(3s)-3-({6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl}amino)pyrrolidin-1-yl]propan-1-one (1/1)
1. Leniolisib
| Molecular Weight | 548.5 g/mol |
|---|---|
| Molecular Formula | C21H28F3N6O6P |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 161 |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 703 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
