



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
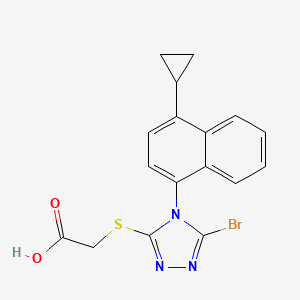

1. ((5-bromo-4-(4-cyclopropyl-1-naphthyl)-4h-1,2,4-triazol-3-yl)sulfanyl)acetic Acid
2. Rdea594
3. Zurampic
1. 878672-00-5
2. Rdea594
3. Rdea 594
4. Zurampic
5. Rdea-594
6. 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-yl)thio)acetic Acid
7. Lesinurad Free Acid
8. (+)-lesinurad
9. (-)-lesinurad
10. Lesinurad, (+)-
11. Lesinurad, (-)-
12. Lesinurad, (4r)-
13. Lesinurad, (4s)-
14. Q59cat99rs
15. 09erp08i3w
16. 73wy698hz7
17. 878672-00-5 (free Acid)
18. 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-ylthio)acetic Acid
19. 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1,2,4-triazol-3-yl]sulfanyl]acetic Acid
20. 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-yl]thio]acetic Acid
21. {[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-yl]sulfanyl}acetic Acid
22. 2-(((4r)-5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4h-1,2,4-triazol-3-yl)thio)acetic Acid
23. 2-(((4s)-5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4h-1,2,4-triazol-3-yl)thio)acetic Acid
24. 2-{[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-yl]sulfanyl}acetic Acid
25. Acetic Acid, 2-((5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4h-1,2,4-triazol-3-yl)thio)-
26. 1890222-25-9
27. 1890222-26-0
28. Acetic Acid, 2-(((4r)-5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4h-1,2,4-triazol-3-yl)thio)-
29. Acetic Acid, 2-(((4s)-5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4h-1,2,4-triazol-3-yl)thio)-
30. Lesinurad [usan]
31. Lesinurad [usan:inn]
32. Unii-09erp08i3w
33. Zurampic (tn)
34. Lesinurad (rdea594
35. Lesinurad [inn]
36. Lesinurad [mi]
37. Lesinurad (usan/inn)
38. Lesinurad (rdea594)
39. Unii-q59cat99rs
40. Lesinurad [who-dd]
41. Schembl842962
42. Unii-73wy698hz7
43. Gtpl7673
44. Lesinurad [orange Book]
45. Chembl2105720
46. Lesinurad, >=98% (hplc)
47. Bdbm37953
48. Chebi:90929
49. Duzallo Component Lesinurad
50. Dtxsid201026091
51. Hms3874m03
52. Amy27876
53. Bcp06435
54. Ex-a1289
55. Us10093631, Compound Lesinurad
56. Mfcd22572730
57. S4640
58. Zinc84757007
59. Akos027327368
60. Ccg-268685
61. Cs-1389
62. Db11560
63. Sb16705
64. Ac-29310
65. As-56014
66. Hy-15258
67. Rdea 594;rdea-594;rdea594
68. Ft-0776044
69. D09921
70. A857828
71. Q21820633
72. (5-bromo-4-(1-cyclopropylnaphthalen-4-yl)-4h-1,2,4-triazol-3-ylthio)acetic Acid
73. 2-(5-bromo-4-(1-cyclopropylnaphthalen-4-yl)-4h-1,2,4-triazol-3-ylthio)acetic Acid
74. 2-[[5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4h-1,2,4-triazol-3-yl]thio]-acetic Acid
75. 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3- Yl)sulfanyl)acetic Acid
76. Rdea 594;sodium 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-yl)thio)acetate
| Molecular Weight | 404.3 g/mol |
|---|---|
| Molecular Formula | C17H14BrN3O2S |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 402.99901 g/mol |
| Monoisotopic Mass | 402.99901 g/mol |
| Topological Polar Surface Area | 93.3 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 479 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For use, in combination with a xanthine oxidase inhibitor, for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone.
FDA Label
Zurampic, in combination with a xanthine oxidase inhibitor, is indicated in adults for the adjunctive treatment of hyperuricaemia in gout patients (with or without tophi) who have not achieved target serum uric acid levels with an adequate dose of a xanthine oxidase inhibitor alone.
Dose-dependent reductions in serum uric acid levels and increases in urinary uric acid excretion have been observed following single and multiple oral doses of lesinurad.
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
Uricosuric Agents
Gout suppressants that act directly on the renal tubule to increase the excretion of uric acid, thus reducing its concentrations in plasma. (See all compounds classified as Uricosuric Agents.)
M04AB05
M - Musculo-skeletal system
M04 - Antigout preparations
M04A - Antigout preparations
M04AB - Preparations increasing uric acid excretion
M04AB05 - Lesinurad
Absorption
Oral lesinurad is rapidly absorbed, reaching maximum plasma concentrations (Cmax) within 14 h following the administration a single 200 mg dose (in either the fed or fasted state).
Route of Elimination
Within 7 days following single dosing of radiolabeled lesinurad, 63% of administered radioactive dose was recovered in urine and 32% of administered radioactive dose was recovered in feces. Most of the radioactivity recovered in urine (> 60% of dose) occurred in the first 24 hours. Unchanged lesinurad in urine accounted for approximately 30% of the dose.
Volume of Distribution
The mean steady state volume of distribution of lesinurad was approximately 20 L following intravenous dosing.
Lesinurad undergoes oxidative metabolism mainly via the polymorphic cytochrome P450 CYP2C9 enzyme.
Lesinurad inhibits the activity of uric acid transporter 1 (URAT1) and organic anion transporter 4 (OAT4). URAT1 is a major transporter enzyme responsible for reuptake of uric acid from the renal tubules; inhibition of URAT1 function thereby increases excretion of uric acid.








