

Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
FDF
0
Australia

0
South Africa

0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
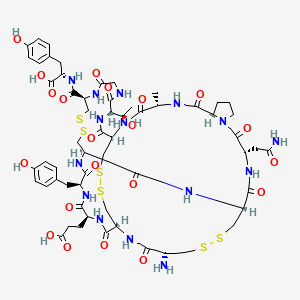

1. Asp-0456
2. Asp0456
3. Linaclotide Acetate
4. Linzess
5. Md-1100
6. Md-1100 Acetate
1. Linzess
2. 851199-59-2
3. Chebi:68551
4. Md-1100
5. Constella
6. Cys Cys Glu Tyr Cys Cys Asn Pro Ala Cys Thr Gly Cys Tyr (disulfide Bridge: 1-6; 2-10; 5-13)
7. Unii-n0txr0xr5x
8. Linaclotide [usan:inn]
9. Hsdb 8224
10. Asp0456
11. Constella (tn)
12. Asp 0456
13. Asp-0456
14. Linzess (tn)
15. Linzess Ammonium Salt
16. Linaclotide Ammonium Salt
17. Linaclotide (jan/usan)
18. N0txr0xr5x
19. Gtpl5017
20. Chembl3301675
21. Schembl13114620
22. Dtxsid90234256
23. Glxc-13151
24. Ex-a6257
25. Db08890
26. Ncgc00389841-01
27. L-tyrosine, L-cysteinyl-l-cysteinyl-l-alpha-glutamyl-l-tyrosyl-l-cysteinyl-l-cysteinyl-l-asparaginyl-l-prolyl-l-alanyl-l-cysteinyl-l-threonylglycyl-l-cysteinyl-, Cyclic (1->6),(2->10),(5->13)-tris(disulfide)
28. D09355
29. Q3832559
30. L-cysteinyl-l-cysteinyl-l-alpha-glutamyl-l-tyrosyl-l-cysteinyl-l-cysteinyl-l-asparaginyl-l-prolyl-l-alanyl-l-cysteinyl-l-threonylglycyl-l-cysteinyl-l-tyrosine Cyclic (1->6),(2->10),(5->13)-tris(disulfide)
| Molecular Weight | 1526.8 g/mol |
|---|---|
| Molecular Formula | C59H79N15O21S6 |
| XLogP3 | -6.8 |
| Hydrogen Bond Donor Count | 19 |
| Hydrogen Bond Acceptor Count | 28 |
| Rotatable Bond Count | 13 |
| Exact Mass | 1525.3899216 g/mol |
| Monoisotopic Mass | 1525.3899216 g/mol |
| Topological Polar Surface Area | 726 Ų |
| Heavy Atom Count | 101 |
| Formal Charge | 0 |
| Complexity | 3030 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Linzess |
| PubMed Health | Linaclotide (By mouth) |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | LINZESS (linaclotide) is a guanylate cyclase-C agonist. Linaclotide is a 14-amino acid peptide with the following chemical name: L-cysteinyl-L-cysteinyl-L-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-L-asparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threon... |
| Active Ingredient | Linaclotide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 290mcg; 145mcg |
| Market Status | Prescription |
| Company | Forest Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Linzess |
| PubMed Health | Linaclotide (By mouth) |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | LINZESS (linaclotide) is a guanylate cyclase-C agonist. Linaclotide is a 14-amino acid peptide with the following chemical name: L-cysteinyl-L-cysteinyl-L-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-L-asparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threon... |
| Active Ingredient | Linaclotide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 290mcg; 145mcg |
| Market Status | Prescription |
| Company | Forest Labs |
Linzess (linaclotide) is indicated in adults for the treatment of irritable bowel syndrome with constipation (IBS-C). /Included in US product label/
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
Linzess is indicated in adults for the treatment of chronic idiopathic constipation (CIC). /Included in US product label/
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
/BOXED WARNING/ WARNING: PEDIATRIC RISK. Linzess is contraindicated in pediatric patients up to 6 years of age; in nonclinical studies, administration of a single, clinically relevant adult oral dose of linaclotide caused deaths due to dehydration in young juvenile mice. Avoid use of Linzess in pediatric patients 6 through 17 years of age. The safety and efficacy of Linzess has not been established in pediatric patients under 18 years of age.
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
Linaclotide is contraindicated in infants and children younger than 6 years of age and should be avoided in children and adolescents 6-17 years of age. Safety and efficacy of linaclotide in pediatric patients have not been established, and the drug has caused deaths in toxicology studies in juvenile mice 1-3 weeks of age (approximately equivalent to infants younger than 2 years of age). The deaths in young juvenile mice occurred following 1 or 2 doses of linaclotide 10 ug/kg administered once daily beginning on postnatal day 7, single oral doses of 100 ug/kg on day 14, and single oral doses of 600 ug/kg on day 21. Although no deaths occurred in juvenile mice 6 weeks of age (approximately equivalent to adolescents 12-17 years of age) receiving linaclotide 20,000 ug/kg daily for 28 days, use of the drug in children and adolescents 6-17 years of age should be avoided because of the deaths reported in younger mice and the lack of safety and efficacy data in pediatric patients.1 No data are available for mice between 3 and 6 weeks of age.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2988
Severe diarrhea was reported in clinical trials in 2% of patients receiving linaclotide for treatment of either irritable bowel syndrome (IBS) with constipation or chronic idiopathic constipation. If severe diarrhea occurs, treatment with the drug should be interrupted or discontinued.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2988
It is not known whether linaclotide is distributed into human milk. Although plasma concentrations of linaclotide and its active metabolite are not measurable following oral administration at recommended dosages, caution is advised when linaclotide is administered to nursing women.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2988
For more Drug Warnings (Complete) data for Linaclotide (10 total), please visit the HSDB record page.
Treatment of irritable bowel syndrome (IBS) with constipation and chronic idiopathic constipation.
FDA Label
Constella is indicated for the symptomatic treatment of moderate to severe irritable-bowel syndrome with constipation (IBS-C) in adults.
Changes in the appearance and consistency of stools as measured by the Bristol Stool Form Scale (BSFS) have been noted after taking linaclotide.
Guanylyl Cyclase C Agonists
Compunds that bind to and activate GUANYLYL CYCLASE-C RECEPTORS. (See all compounds classified as Guanylyl Cyclase C Agonists.)
A06AX04
A06AX04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AX - Other drugs for constipation
A06AX04 - Linaclotide
Absorption
When taken orally, linaclotide is not absorbed into the systemic. No detectable levels of linaclotide or its active metabolite were noted after doses of 125 mcg or 290 mcg were administered.
Route of Elimination
Linaclotide is eliminated fecally (3 - 5% as active metabolites). However most of the dose undergoes proteolysis (processes include reduction of disulfide bonds) in the intestine before being excreted via feces.
Volume of Distribution
Given that linaclotide plasma concentrations following therapeutic oral doses are not measurable, linaclotide is expected to be minimally distributed to tissues.
Given that linaclotide plasma concentrations following therapeutic oral doses are not measurable, linaclotide is expected to be minimally distributed to tissues.
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
Active peptide recovery in the stool samples of fed and fasted subjects following the daily administration of 290 mcg of Linzess for seven days averaged about 5% (fasted) and about 3% (fed) and virtually all as the active metabolite.
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
Linzess is minimally absorbed with low systemic availability following oral administration. Concentrations of linaclotide and its active metabolite in plasma are below the limit of quantitation after oral doses of 145 ug or 290 ug were administered. Therefore, standard pharmacokinetic parameters such as area under the curve (AUC), maximum concentration (Cmax), and half-life cannot be calculated.
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
It is not known whether linaclotide is distributed into human milk.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2988
For more Absorption, Distribution and Excretion (Complete) data for Linaclotide (8 total), please visit the HSDB record page.
Linaclotide is metabolized within the gastrointestinal tract to its principal, active metabolite, MM-419447, by loss of the terminal tyrosine moiety. Both linaclotide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids.
The metabolism of linaclotide was investigated in a set of experiments, predominantly in rodents. Linaclotide is metabolised in the intestine by immediate break down of the disulfide bridges which prone linaclotide to further digestion by the enzymes present in the gastrointestinal environment. Several breakdown products containing 3-13 amino acids have been identified. Only one metabolite, MM-419447, was shown to be pharmacodynamic active.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Constella (Linaclotide) p.18 (2012). Available from, as of March 18, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002490/WC500135624.pdf
Linaclotide is metabolized within the gastrointestinal tract to its principal, active metabolite by loss of the terminal tyrosine moiety. Both linaclotide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids.
NIH; DailyMed. Current Medication Information for Linzess (Linaclotide) Capsule, Gelatin Coated (Revised: July 2014). Available from, as of March 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09beda19-56d6-4a56-afdc-9a77b70b2ef3
... We examined the metabolic stability of linaclotide in conditions that mimic the gastrointestinal tract and characterized the metabolite MM-419447 (CCEYCCNPACTGC), which contributes to the pharmacologic effects of linaclotide. Systemic exposure to these active peptides is low in rats and humans, and the low systemic and portal vein concentrations of linaclotide and MM-419447 observed in the rat confirmed both peptides are minimally absorbed after oral administration. Linaclotide is stable in the acidic environment of the stomach and is converted to MM-419447 in the small intestine. The disulfide bonds of both peptides are reduced in the small intestine, where they are subsequently proteolyzed and degraded. After oral administration of linaclotide, <1% of the dose was excreted as active peptide in rat feces and a mean of 3-5% in human feces; in both cases MM-419447 was the predominant peptide recovered. MM-419447 exhibits high-affinity binding in vitro to T84 cells, resulting in a significant, concentration-dependent accumulation of intracellular cyclic guanosine-3',5'-monophosphate (cGMP). In rat models of gastrointestinal function, orally dosed MM-419447 significantly increased fluid secretion into small intestinal loops, increased intraluminal cGMP, and caused a dose-dependent acceleration in gastrointestinal transit. These results demonstrate the importance of the active metabolite in contributing to linaclotide's pharmacology.
PMID:23090647 Busby RW et al; J Pharmacol Exp Ther 344 (1): 196-206 (2013)
Because linaclotide is not systemically absorbed, half life cannot be calculated.
Two male and two female monkeys were intravenously dosed for seven consecutive days with 15 mg/kg/day linaclotide. ... /The/ mean half life was approximately 1.5 hr on day 1 and 7 for both genders.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Constella (Linaclotide) p.19 (2012). Available from, as of March 18, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002490/WC500135624.pdf
Linaclotide is an agonist of guanylate cyclase-C (GC-C). Once linaclotide and its active metabolite binds to GC-C, it has local effect on the luminal surface of the intestinal epithelium. Activation of GC-C by linaclotide results in the intra- and extracellular increase of cyclic guanosine monophosphate concentrations (cGMP). This elevation of cGMP levels stimulates the secretion of chloride and bicarbonate into the intestinal lumen via activation of cystic fibrosis transmembrane conductance regulator (CFTR) ion channel. Ultimately, linaclotide helps patients with IBS (especially with constipation) as GI transit is accelerated and the release of intestinal fluid is increased. In animal models, a decrease in visceral pain after administration of linaclotide may be observed. A decrease in the activity of pain-sensing nerves occurs as a result of an increase in extracellular cGMP.
Activation of guanylate cyclase-C (GC-C) expressed predominantly on intestinal epithelial cells by guanylin, uroguanylin or the closely related GC-C agonist peptide, linaclotide, stimulates generation, and release of cyclic guanosine-3',5'-monophosphate (cGMP). Evidence that the visceral analgesic effects of linaclotide are mediated by a novel, GC-C-dependent peripheral sensory mechanism was first demonstrated in animal models of visceral pain. Subsequent studies with uroguanylin or linaclotide have confirmed the activation of a GC-C/cGMP pathway leading to increased submucosal cGMP mediated by cGMP efflux pumps, which modulates intestinal nociceptor function resulting in peripheral analgesia. These effects can be reproduced by the addition of exogenous cGMP and support a role for GC-C/cGMP signaling in the regulation of visceral sensation, a physiological function that has not previously been linked to the GC-C/cGMP pathway. Notably, targeting the GC-C/cGMP pathway for treatment of gastrointestinal pain and abdominal sensory symptoms has now been validated in the clinic. ...
PMID:24795564 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997039 Hannig G et al; Front Mol Neurosci 7: 31 (2014)
Linaclotide, a synthetic 14-amino acid peptide, is a potent and selective guanylate cyclase-C (GC-C) agonist with visceral analgesic and secretory activities. This first-in-class orally active peptide is structurally related to the guanylin peptide family, which is involved in the regulation of fluid homeostasis and bowel function of the GI tract. Both linaclotide and its active metabolite bind to GC-C and act locally on the luminal surface of the intestinal epithelium. Activation of GC-C results in an increase in both intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP). Elevation in intracellular cGMP stimulates secretion of chloride and bicarbonate into the intestinal lumen, through activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, resulting in increased intestinal fluid and accelerated transit. Linaclotide has been shown to both accelerate GI transit and reduce intestinal pain. The linaclotide-induced reduction in visceral pain is thought to be mediated by increased extracellular cGMP, which was shown to decrease the activity of pain-sensing nerves.
Health Canada; Product Monograph for Constella (Linaclotide), Drug Identification Number (DIN): 02417162 p.12 (Date of Preparation: May 12, 2014). Available from, as of March 18, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
Linaclotide is a minimally absorbed agonist of guanylate cyclase-C (GUCY2C or GC-C) that reduces symptoms associated with irritable bowel syndrome with constipation (IBS-C). Little is known about the mechanism by which linaclotide reduces abdominal pain in patients with IBS-C. We determined the effects of linaclotide on colonic sensory afferents in healthy mice and those with chronic visceral hypersensitivity. We assessed pain transmission by measuring activation of dorsal horn neurons in the spinal cord in response to noxious colorectal distention. Levels of Gucy2c messenger RNA were measured in tissues from mice using quantitative reverse transcription polymerase chain reaction and in situ hybridization. We used human intestinal cell lines to measure release of cyclic guanosine-3',5'-monophosphate (cGMP) by linaclotide. We performed a post-hoc analysis of data from a phase III, double-blind, parallel-group study in which 805 patients with IBS-C were randomly assigned to groups given an oral placebo or 290 ug linaclotide once daily for 26 weeks. We quantified changes in IBS-C symptoms, including abdominal pain. In mice, linaclotide inhibited colonic nociceptors with greater efficacy during chronic visceral hypersensitivity. Intra-colonic administration of linaclotide reduced signaling of noxious colorectal distention to the spinal cord. The colonic mucosa, but not neurons, was found to express linaclotide's target, GC-C. The downstream effector of GC-C, cGMP, was released after administration of linaclotide and also inhibited nociceptors. The effects of linaclotide were lost in Gucy2c(-/-) mice and prevented by inhibiting cGMP transporters or removing the mucosa. During 26 weeks of linaclotide administration, a significantly greater percentage of patients (70%) had at least a 30% reduction in abdominal pain compared with patients given placebo (50%). We have identified an analgesic mechanism of linaclotide: it activates GC-C expressed on mucosal epithelial cells, resulting in the production and release of cGMP. This extracellular cGMP acts on and inhibits nociceptors, thereby reducing nociception. We also found that linaclotide reduces chronic abdominal pain in patients with IBS-C.
PMID:23958540 Castro J et al; Gastroenterology 145 (6): 1334-46.e1-11 (2013)
Linaclotide is a guanlylate cyclase-C (GC-C) receptor agonist. GC-C receptor is found in the luminal aspect of intestinal epithelium and dopamine neurons in the brain, and is a key receptor for heat-stable enterotoxins that are responsible for acute secretory diarrhea. Linaclotide is structurally related to the guanylin peptide family, which is involved in the regulation of intestinal fluid homeostasis and bowel function, and includes the hormones guanylin and uroguanlyin. Linaclotide, similarly to guanylin and uroguanylin, is able to increase intracellular concentrations of the second messenger cyclic guanosine monophosphate (cGMP) through activation of the GC-C receptor, located on the apical surface of epithelial cells throughout the intestine. The presence of intracellular cGMP triggers a signal transduction cascade that leads to the activation of the cystic fibrosis transmembrane conductance regulator (CFTR) through its cGMP-dependent phosphorylation by protein kinase G II (PKG II). CFTR activation causes secretion of chloride and bicarbonate into the intestinal lumen, causing an increase in fluid secretion and acceleration of GI transit. cGMP is also transported out of the cell into the intestinal lumen and submucosa, modulating the activity of local afferent nerve fibers and causing a reduced visceral pain.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Constella (Linaclotide) p.16 (2012). Available from, as of March 18, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002490/WC500135624.pdf
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24992
Submission : 2011-05-27
Status : Active
Type : II

Registration Number : 231MF10090
Registrant's Address : 2075 North 55th Street Boulder, Colorado 80301-2880, U.S. S. A
Initial Date of Registration : 2019-04-16
Latest Date of Registration : --

NDC Package Code : 12869-110
Start Marketing Date : 2012-08-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2016-12-23
Pay. Date : 2016-02-23
DMF Number : 30266
Submission : 2016-11-16
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2015-12-04
Pay. Date : 2015-09-18
DMF Number : 29708
Submission : 2015-09-22
Status : Active
Type : II
Date of Issue : 2022-06-10
Valid Till : 2019-07-22
Written Confirmation Number : WC-0443-A1-2
Address of the Firm :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : LINZESS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 145MCG
Packaging :
Approval Date : 2012-08-30
Application Number : 202811
Regulatory Info : RX
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : LINZESS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 290MCG
Packaging :
Approval Date : 2012-08-30
Application Number : 202811
Regulatory Info : RX
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : LINZESS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 72MCG
Packaging :
Approval Date : 2017-01-25
Application Number : 202811
Regulatory Info : RX
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Kapsel, hard
Dosage Strength : 290 mikrog
Packaging : Boks 28item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Kapsel, hard
Dosage Strength : 290 mikrog
Packaging : Boks 90item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Constella
Dosage Form : Kaps
Dosage Strength : 290mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Constella
Dosage Form : Kaps
Dosage Strength : 290mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : CONSTELLA
Dosage Form : CAPSULE
Dosage Strength : 145MCG
Packaging :
Approval Date :
Application Number : 2417162
Regulatory Info : Prescription
Registration Country : Canada
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : CONSTELLA
Dosage Form : CAPSULE
Dosage Strength : 290MCG
Packaging :
Approval Date :
Application Number : 2417170
Regulatory Info : Prescription
Registration Country : Canada
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : CONSTELLA
Dosage Form : CAPSULE
Dosage Strength : 72MCG
Packaging : Apr-30
Approval Date :
Application Number : 2469510
Regulatory Info : Prescription
Registration Country : Canada
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : LINZESS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 145MCG
Approval Date : 2012-08-30
Application Number : 202811
RX/OTC/DISCN : RX
RLD : Yes
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : LINZESS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 290MCG
Approval Date : 2012-08-30
Application Number : 202811
RX/OTC/DISCN : RX
RLD : Yes
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : LINZESS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 72MCG
Approval Date : 2017-01-25
Application Number : 202811
RX/OTC/DISCN : RX
RLD : Yes
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : LINACLOTIDE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 72MCG
Approval Date :
Application Number : 211255
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LINACLOTIDE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 145MCG
Approval Date : 2023-02-07
Application Number : 209611
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LINACLOTIDE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 290MCG
Approval Date : 2023-02-07
Application Number : 209611
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LINACLOTIDE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 145MCG
Approval Date : 2021-02-09
Application Number : 209564
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LINACLOTIDE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 290MCG
Approval Date : 2021-02-09
Application Number : 209564
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Kapsel, hard
Dosage Strength : 290 mikrog
Packaging : Boks 28item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Kapsel, hard
Dosage Strength : 290 mikrog
Packaging : Boks 90item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Constella
Dosage Form : Kaps
Dosage Strength : 290mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Constella
Dosage Form : Kaps
Dosage Strength : 290mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Capsule, hard
Dosage Strength : 290 mcg
Packaging : Box
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Capsule, hard
Dosage Strength : 290 mcg
Packaging : Box
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Granberg
Dosage Form : HARD CAPSULES
Dosage Strength : 290 MICROGRAMS
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Constella
Dosage Form : Kapsel, hard
Dosage Strength : 290 mikrog
Packaging : Boks 28item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 72MCG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 72MCG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 145MCG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 145MCG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 290MCG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 290MCG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Argentina
Brand Name : Lilla
Dosage Form : Capsule
Dosage Strength : 145MG
Packaging : 28 Caps
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Argentina

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 28 Caps
Regulatory Info :
Dosage : Capsule
Dosage Strength : 145MG
Brand Name : Lilla
Approval Date :
Application Number :
Registration Country : Argentina

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Argentina
Brand Name : Lilla
Dosage Form : Capsule
Dosage Strength : 290MG
Packaging : 28 Caps
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Argentina

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 28 Caps
Regulatory Info :
Dosage : Capsule
Dosage Strength : 290MG
Brand Name : Lilla
Approval Date :
Application Number :
Registration Country : Argentina

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
