
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
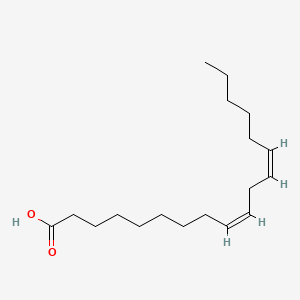

1. 9 Trans,12 Trans Octadecadienoic Acid
2. 9,12 Octadecadienoic Acid
3. 9,12-octadecadienoic Acid
4. 9-trans,12-trans-octadecadienoic Acid
5. Acid, 9,12-octadecadienoic
6. Cis,cis-9,12-octadecadienoic Acid
7. Linoelaidic Acid
8. Linoelaidic Acid, (e,z)-isomer
9. Linoleate
10. Linoleic Acid, (e,e)-isomer
11. Linoleic Acid, (z,e)-isomer
12. Linoleic Acid, (z,z)-isomer
13. Linoleic Acid, (z,z)-isomer, 14c-labeled
14. Linoleic Acid, Ammonium Salt, (z,z)-isomer
15. Linoleic Acid, Calcium Salt, (z,z)-isomer
16. Linoleic Acid, Potassium Salt, (z,z)-isomer
17. Linoleic Acid, Sodium Salt, (e,e)-isomer
18. Linoleic Acid, Sodium Salt, (z,z)-isomer
19. Linolelaidic Acid
20. Trans,trans-9,12-octadecadienoic Acid
1. 60-33-3
2. Linolic Acid
3. (9z,12z)-octadeca-9,12-dienoic Acid
4. Telfairic Acid
5. Cis,cis-linoleic Acid
6. Linoleate
7. 9,12-linoleic Acid
8. Grape Seed Oil
9. Cis,cis-9,12-octadecadienoic Acid
10. Emersol 315
11. Unifac 6550
12. Polylin 515
13. Cis-9,cis-12-octadecadienoic Acid
14. 9z,12z-linoleic Acid
15. (z,z)-9,12-octadecadienoic Acid
16. 9z,12z-octadecadienoic Acid
17. Extra Linoleic 90
18. 9-cis,12-cis-linoleic Acid
19. Emersol 310
20. All-cis-9,12-octadecadienoic Acid
21. Polylin No. 515
22. Oils, Grape
23. Linoleic
24. Acide Linoleique
25. 9,12-octadecadienoic Acid (9z,12z)-
26. 9-cis,12-cis-octadecadienoic Acid
27. Alpha-linoleic Acid
28. Acido Linoleico
29. 8024-22-4
30. 9,12-octadecadienoic Acid
31. Leinoleic Acid
32. (9z,12z)-octadecadienoic Acid
33. Ronacare Asc 3
34. 9,12-octadecadienoic Acid (z,z)-
35. Acide Cis-linoleique
36. (9z,12z)-9,12-octadecadienoic Acid
37. Chebi:17351
38. Cis-9, Cis-12-octadecadienoic Acid
39. Cis-delta(9,12)-octadecadienoic Acid
40. Nsc-281243
41. 9kjl21t0qj
42. Chembl267476
43. 9-cis,12-cis-octadecadienoate
44. C18:2
45. Cis,cis-linoleate
46. Ncgc00091049-04
47. C18:2 (n-6)
48. Fema No. 3380, Linoleic Acid-
49. Vespula Pensylvanica B708568k063
50. Dsstox_cid_5505
51. Dsstox_rid_77814
52. Dsstox_gsid_25505
53. Mfcd00064241
54. Oils, Grape Seed
55. Cas-60-33-3
56. 8016-21-5
57. Ccris 650
58. 14c-linoleic Acid
59. Linoleic Acid, Pure
60. Hsdb 5200
61. (14c)-linoleic Acid
62. Sr-01000944790
63. Cis-delta9,12-octadecadienoic Acid
64. Einecs 200-470-9
65. Unii-9kjl21t0qj
66. 9,12-octadecadienoic Acid, (z,z)-
67. Nsc 281243
68. (14c)alpha-linolenic Acid
69. Acidelinoleique
70. 7552p0k6pn
71. Linolate
72. Grapeseed Oil
73. Linoleic-acid
74. Leinolic Acid
75. Ai3-11132
76. Trans-9,trans-12-octadecadienoic Acid
77. 9z,12z-linoleate
78. Linoleic Acid 315
79. N-6,9 All-cis
80. Linoleic Acid, 95%
81. 9z,12z-octadecadienoate
82. 9-cis,12-cis-linoleate
83. Linoleic Acid, >=95%
84. Linoleic Acid, >=99%
85. Bmse000497
86. Bmse000604
87. Epitope Id:117705
88. Linoleic Acid [mi]
89. Linoleic And Linolenic Acids
90. Schembl7067
91. (9z,12z)-9,12-octadecadienoic-1-13c Acid
92. Cis,12-octadecadienoic Acid
93. Linoleic Acid [fcc]
94. Pamolyn 125 (salt/mix)
95. Cis-d9,12-octadecadienoate
96. Bspbio_001374
97. Linoleic Acid [hsdb]
98. Linoleic Acid [inci]
99. 80969-37-5
100. Ccris 652
101. Linoleic Acid [vandf]
102. Unii-7552p0k6pn
103. Linoleic Acid [mart.]
104. All-cis-9,12-octadecadienoate
105. Bml3-c03
106. Cis-9,cis-12-octadecadienoate
107. Gtpl1052
108. Linoleic Acid, >=95%, Fg
109. Linoleic Acid, Puriss., 90%
110. Delta9,12-octadecadienoic Acid
111. Linoleic Acid [who-dd]
112. (z,z)-9,12-octadecadienoate
113. Dtxsid2025505
114. Acon1_000270
115. Bdbm22231
116. C18:2, N-6,9 All-cis
117. Cis-d9,12-octadecadienoic Acid
118. Linoleic Acid, >=93% (gc)
119. Chebi:137735
120. 9,12-octadecadienoic Acid, (z,z)-, Labeled With Carbon-14
121. Hms1361e16
122. Hms1791e16
123. Hms1989e16
124. Hms3402e16
125. Hms3649f07
126. Hms3886f05
127. Linoleic Acid, Analytical Standard
128. 9,12-octadecadienoic Acid (van)
129. Hy-n0729
130. Zinc4474613
131. Tox21_111067
132. Tox21_202171
133. Tox21_303080
134. (9z,12z)-9,12-octadecadienoate
135. C18:2 9c
136. Cis,cis-octadeca-9,12-dienoic Acid
137. Cis-9,cis-12-octadecadienoic Acid,
138. Lmfa01030120
139. Nsc281243
140. S5821
141. (z,z)-octadeca-9, 12-dienoic Acid
142. 9,12-octadecadienoic Acid, (e,e)-
143. 9,12-octadecadienoic Acid, Cis,cis-
144. Akos015951293
145. Db14104
146. Rans, Trans-9,12-octadecadienoic Acid
147. 9-(z), 12-(z)-octadecadienoic Acid
148. Cis-.delta.9,12-octadecadienoic Acid
149. Idi1_033844
150. Ncgc00091049-01
151. Ncgc00091049-02
152. Ncgc00091049-03
153. Ncgc00091049-05
154. Ncgc00091049-06
155. Ncgc00091049-07
156. Ncgc00257024-01
157. Ncgc00259720-01
158. Vitamin F Component Linoleic Acid
159. 12c Omega6 Todos Cis-9,12-octadienoico
160. Ac-33770
161. As-12672
162. Bp-31121
163. Fa(18:2(9z,12z))
164. Linoleic Acid, Technical, 60-74% (gc)
165. Octadeca-9,12-dienoic Acid, (cis,cis)-
166. Ai3-36448
167. 9,z)-
168. Cs-0009742
169. L0053
170. L0124
171. C01595
172. 9, (z)-
173. A832696
174. Q407426
175. Sr-01000944790-1
176. Sr-01000944790-3
177. Brd-k08973992-001-03-9
178. C18:2 9c, 12c Omega6 Todos Cis-9,12-octadienoico
179. Linoleic Acid (constituent Of Spirulina) [dsc]
180. 5ce5e1f3-8859-4c5b-9afe-e44a7076df6e
181. Linoleic Acid (constituent Of Flax Seed Oil) [dsc]
182. Linoleic Acid (constituent Of Saw Palmetto) [dsc]
183. Linoleic Acid (constituent Of Borage Seed Oil) [dsc]
184. Linoleic Acid, Liquid, Bioreagent, Suitable For Cell Culture
185. Linoleic Acid (c18:2) (constituent Of Krill Oil) [dsc]
186. Linoleic Acid, 2.0 Mg/ml In Ethanol, Certified Reference Material
187. Cis,cis-9,12-octadecadienoic Acid; Linoleate; Emersol315; Linoleic; Unifac6550;
188. 30175-49-6
189. 85594-37-2
190. Lin
191. Linoleic Acid, Pharmagrade, Manufactured Under Appropriate Controls For Use As A Raw Material In Pharma Or Biopharmaceutical Production
| Molecular Weight | 280.4 g/mol |
|---|---|
| Molecular Formula | C18H32O2 |
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 14 |
| Exact Mass | 280.240230259 g/mol |
| Monoisotopic Mass | 280.240230259 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 267 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ To determine the effect of low doses of linoleic acid and calcium on prostaglandin (PG) levels and the efficacy of this treatment in the prevention of preeclampsia. In a randomized, double-blind, placebo-controlled study we treated 86 primigravidas with risk factors for preeclampsia (high biopsychosocial risk [above 3 points], positive roll-over test, and high mean blood pressure [above 85 mmHg)] with daily doses of either 450 mg linoleic acid and 600 mg calcium (n=43) or 450 mg starch and 600 mg lactose placebo (n=43) during the third trimester of pregnancy. Four women in the experimental group (9.3%) developed preeclampsia compared with 16 (37.2%) controls (relative risk 0.25, 95% confidence interval 0.09, 0.69, P < .001). The median serum levels of PGE2 after 4 weeks of treatment increased by 106% in the experimental group (P=.03) and decreased by 33% in the control group (P=.02). The median ratio between thromboxane B2 and PGE2 decreased by 40% in the experimental group (P=.02) and increased by 18% in the control group (P=.14). No significant differences were observed in the median ratio between thromboxane B2 and 6-keto PGF1alpha in either group. No serious maternal or neonatal side effects of treatment occurred in either group. The administration of low daily doses of linoleic acid and calcium during the third trimester of pregnancy reduced the incidence of preeclampsia significantly in women at high risk, possibly by correcting the PGE2 levels.
PMID:9540946 Herrera JA et al; Obstet Gynecol 91 (4): 585-90 (1998)
/EXPL THER/ Abdominal obesity is strongly related to metabolic disorders. Recent research suggests that dietary conjugated linoleic acid (CLA) reduces body fat and may improve metabolic variables in animals. The metabolic effects of CLA in abdominally obese humans have not yet been tested. To investigate the short-term effect of CLA on abdominal fat and cardiovascular risk factors in middle-aged men with metabolic disorders, twenty-five abdominally obese men (waist-to-hip ratio (WHR), 1.05+/-0.05; body mass index (BMI), 32+/-2.7 kg/m(2) (mean+/-s.d.)) who were between 39 and 64-y-old participated in a double-blind randomised controlled trial for 4 weeks. Fourteen men received 4.2 g CLA/day and 10 men received a placebo. The main endpoints were differences between the two groups in sagittal abdominal diameter (SAD), serum cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, free fatty acids, glucose and insulin. At baseline, there were no significant differences between groups in anthropometric or metabolic variables. After 4 weeks there was a significant decrease in SAD (cm) in the CLA group compared to placebo (P=0.04, 95% CI; -1.12, -0.02). Other measurements of anthropometry or metabolism showed no significant differences between the groups. These results indicate that CLA supplementation for 4 weeks in obese men with the metabolic syndrome may decrease abdominal fat, without concomitant effects on overall obesity or other cardiovascular risk factors. /Conjugated linoleic acid/
PMID:11477497 Riserus U et al; Int J Obes Relat Metab Disord 25 (8): 1129-35 (2001)
/EXPL THER/ Conjugated linoleic acid (CLA) refers to a group of positional and geometric isomers of the omega-6 essential fatty acid linoleic acid (cis-9, cis-12, octadecadienoic acid). In humans evidence is currently ambiguous as to whether CLA supplementation has a significant effect on body composition. Despite favorable changes in lipid levels in animal models, a beneficial effect in humans has not yet been established. While some of the changes reported are consistent with an improved lipid profile, declines in HDL and increases in lipoprotein (a) have also been observed in some subjects. Available evidence suggests CLA supplementation has no impact on immune system performance in healthy subjects. /Conjugated linoleic acid/
PMID:11578253 Kelly GS; Altern Med Rev 6 (4): 367-82 (2001)
/EXPL THER/ Melasma is an acquired symmetric hypermelanosis characterized by irregular light-to gray-brown macules and patches on sun-exposed areas. Many therapeutic agents are available but are unsatisfactory. Recently, it has been demonstrated that lincomycin (LM) and linoleic acid (LA) can inhibit melanogenesis in vitro. /The authors/ investigated the clinical efficacy of topical application of LM and LA in combination with betamethasone valerate (BV) in melasma patients. Forty-seven Korean female adults with clinically diagnosed melasma were enrolled in a 6-week, double-blind, randomized clinical trial. Patients were treated with one application of the vehicle (group A), 2% LM mixed with 0.05% BV (group B), or 2% LM mixed with 0.05% BV and 2% LA (group C) on the face every night. Determination of efficacy was based on the Melasma Area and Severity Index (MASI) score and objective assessment (no effect, mild, moderate, or excellent) at intervals of 2 weeks until the end of the study at 6 weeks. After 6 weeks, in comparison with the pre-treatment MASI score, the average MASI score of group C decreased to 68.9%, compared with 98% in group A (p<0.05) and 85.4% in group B. There was no statistically significant difference between group A and group B. Seven patients (43.7%) in group C revealed more than moderate improvement in objective assessment, compared with none in group A and two patients (12.5%) in group B. There were no significant side effects. Topical application of linoleic acid is considered to be effective in the treatment of melasma patients.
PMID:12172049 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3054896 Lee MH et al; J Korean Med Sci 17 (4): 518-23 (2002)
For more Therapeutic Uses (Complete) data for LINOLEIC ACID (11 total), please visit the HSDB record page.
Because of lack of long-term safety data, /conjugated linoleic acid/ CLA supplements should be avoided by children, pregnant women and nursing mothers. /Conjugated linoleic acid
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 160
At doses of up to 2 g /Conjugated linoleic acid/ daily, occasional gastointestinal complaints, such as nausea, have been noted. /Conjugated linoleic acid/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 160
1. Practically nontoxic: probable oral lethal dose (human) above 15 g/kg, more than 1 qt (2.2 lb) for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-199
... The objectives of the study were to 1) use the whole body fatty acid balance method to quantify whole-body concentrations of linoleate in humans, 2) estimate the distribution of linoleate between adipose and lean tissue, and 3) assess the effect of weight loss on linoleate stores and beta-oxidation in obese humans. Nine healthy obese men underwent supervised weight loss for 112 d (16 wk). Magnetic resonance imaging data and fatty acid profiles from fat biopsies were both used to determine linoleate stores in adipose and lean tissue and in the whole body. Linoleate beta-oxidation was calculated as intake - (accumulation + excretion). Mean weight loss was 13 kg and linoleate intake was 24 +/- 6 mmol/d over the study period. Whole-body loss of linoleate was 37 +/- 18 mmol/d, or 28% of the level before weight loss. Combining the intake and whole-body loss of linoleate resulted in linoleate beta-oxidation exceeding intake by 2.5-fold during the weight-loss period. All dietary linoleate is beta-oxidized and at least an equivalent amount of linoleate is lost from the body during moderate weight loss in obese men. The method studied permits the assessment of long-term changes in linoleate homeostasis in obese humans and may be useful in determining the risk of linoleate deficiency in other conditions. /Linoleate/
PMID:11273844 Cunnane SC et al; Am J Clin Nutr 73 (4): 709-14 (2001)
Human milk fatty acids vary with maternal dietary fat composition. Hydrogenated dietary oils with trans fatty acids may displace cis n-6 and n-3 unsaturated fatty acids or have adverse effects on their metabolism. The effects of milk trans, n-6, and n-3 fatty acids in breast-fed infants are unclear, although n-6 and n-3 fatty acids are important in infant growth and development. /The authors/ sought to determine the relations between trans and cis unsaturated fatty acids in milk and plasma phospholipids and triacylglycerols of breast-fed infants, and to identify the major maternal dietary sources of trans fatty acids. collected milk from 103 mothers with exclusively breast-fed 2-mo-old infants, blood from 62 infants, and 3-d dietary records from 21 mothers. Results: Mean (+/-SEM) percentages of trans fatty acids were as follows: milk, 7.1 +/- 0.32%; infants' triacylglycerols, 6.5 +/- 0.33%; and infants' phospholipids, 3.7 +/- 0.16%. Milk trans fatty acids, a-linolenic acid (18:3n-3), arachidonic acid (20:4n-6), docosahexaenoic acid (22:6n-3) (P < 0.001), and linoleic acid (18:2n-6) (P = 0.007) were each related to the same fatty acid in infant plasma phospholipids. Milk trans fatty acids were inversely related to milk 18:2n-6 and 18:3n-3, but not to milk or infant plasma 20:4n-6 or 22:6n-3. trans Fatty acids represented 7.7% of maternal total fat intake (2.5% of total energy); the major dietary sources were bakery products and breads (32%), snacks (14%), fast foods (11%), and margarines and shortenings (11%). There were comparable concentrations of trans fatty acids in the maternal diet, breast milk, and plasma triacylglycerols of breast-fed infants. Prepared foods were the major dietary source of trans fatty acids.
Innis SM, King J; Am J Clin Nutr 70 (3): 383-390 (1999)
Some /conjugated linoleic acid/ CLA appears to get incorporated into the phospholipids of cell membranes. /Conjugated linoleic acid/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 159
/OTHER TOXICITY INFORMATION/ The hepatotoxicity of orally administered secondary autoxidation products of linoleic acid in rats was investigated and compared to the effects following administration of a saline solution and linoleic acid as controls. The de novo synthesis of fatty acids was strongly reduced in the secondary products group. The level of nicotine adenine dinucleotide phosphate (NADPH) in the liver significantly decreased whereas that of nicotine adenine dinucleotide (NADH) did not. The activities of glucose 6-phosphate dehydrogenase and phosphogluconate dehydrogenase apparently decreased. The activities of NAD + kinase and NAD + synthetase decreased and that of NAD + nucleosidase increased in the secondary products group. Therefore the depletion of nicotine adenine dinucleotide phosphate can be attributed to the inhibition of two metabolic systems (a nicotine adenine dinucleotide phosphate-supplemental system and a synthetic system of NADP and NAD), and resulted in the reduction of lipogenesis in the liver. /Autooxidation products/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 822
... Linoleic acid stimulated tumor growth because it is converted by hepatoma 7288CTC to the mitogen, 13-hydroxyoctadecadienoic acid (13-HODE). ...
PMID:11377374 Sauer LA et al; Biochem Pharmacol 61 (12): 1455-62 (2001)
13-hydroxyoctadecadienoic acid (13-HODE) synthesis is enhanced by cyclic AMP. Gamma-linolenic acid, a desaturated metabolite of linoleic acid, causes substantial stimulation of 13-HODE synthesis. A fall in gamma-linolenic acid synthesis with age may be related to the age-related fall in 13-HODE formation. /gamma-Linolenic acid/
PMID:9561154 Horrobin DF et al; Adv Exp Med Biol 433: 291-4 (1997)
Linoleic acid has known human metabolites that include Isoleukotoxin and Leukotoxin.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
/The objective of this work was/ to study the gene expression of the resistin and the effects of conjugated linoleic acid on its expression in white adipose tissue of obese rats fed with high fat diet during the formation of insulin resistance. Male Wistar rats were randomly separated in control group, high-fat group and high fat + conjugated linoleic acid (CLA) group (0.75 g, 1.50 g, 3.00 g per 100 g diet weight), using reverse transcription polymerase chain reaction (RT-PCR) technique to measure the expression level of resistin and peroxisome proliferator-activated receptor-gamma (PPARgamma) mRNA expression. The serum insulin and glucose levels of obese rats were (11.11 +/- 2.73) mIU/L, (5.09 +/- 0.66) mmol/L, and supplement of CLA might decrease hyperinsulinemia and hyperglycemia, in CLA group (0.75 g, 1.50 g, 3.00 g per 100 g diet weight) the serum insulin levels were (6.99 +/- 1.77) mIU/L, (7.36 +/- 1.48) mIU/L, (7.85 +/- 1.60) mIU/L, and glucose levels were (4.28 +/- 0.72) mmol/L, (4.18 +/- 0.55) mmol/L, (4.06 +/- 0.63) mmol/L. The expression of resistin in adipose tissue of obese rat fed with high fat diet was increased as compared with those fed with basic diet. CLA might increase the expression of resistin and PPARgamma in adipose tissue of obese rat. The expression of resistin mRNA of obese rat fed with high fat diet was higher than those fed with basic diet, and CLA might improve the insulin resistance in obese rats and possibly upregulate the expression of resistin through activing PPARgamma. /Conjugated linoleic acid/
PMID:15938854 Zhou XR et al; Zhonghua Yu Fang Yi Xue Za Zhi 39 (3): 191-4 (2005)
Conjugated linoleic acid (CLA) is a mixture of positional (e.g. 7,9; 9,11; 10,12; 11,13) and geometric (cis or trans) isomers of octadecadienoic acid. This compound was first shown to prevent mammary carcinogenesis in murine models. Later investigations uncovered a number of additional health benefits, including decreasing atherosclerosis and inflammation while enhancing immune function. The mechanisms of action underlying these biological properties are not clearly understood. The aim of this review is to highlight recent advances in CLA research related to experimental inflammatory bowel disease. In addition, two possible mechanisms of action (i.e. endoplasmic and nuclear) were discussed in detail in the context of enteric inflammatory disorders. Conjugated linoleic acid was first implicated in down-regulating the generation of inducible eicosanoids (i.e. PGE(2) and LTB(4)) involved in early micro-inflammatory events (endoplasmic). More recently, CLA has been shown to modulate the expression of genes regulated by peroxisome proliferator-activated receptors (PPARs; nuclear). In pigs, prolonged dietary CLA treatment stimulated the expression of PPAR-gamma in the muscle. Thus, evidence supporting both mechanistic theories of CLA acting through eicosanoid synthesis and PPAR activity is available. The further understanding of the anti-inflammatory mechanisms of action of CLA may yield novel nutritional therapies for enteric inflammation. /Conjugated linoleic acid/
PMID:12468364 Bassaganya-Riera J et al; Clin Nutr 21 (6): 451-9 (2002)
/Conjugated linoleic acid/ CLA may modulate eicosanoid activity as well as the activity... of tumor necrosis factor-alpha. /Conjugated linoleic acid/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 159

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
31
PharmaCompass offers a list of Linoleic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Linoleic Acid manufacturer or Linoleic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Linoleic Acid manufacturer or Linoleic Acid supplier.
PharmaCompass also assists you with knowing the Linoleic Acid API Price utilized in the formulation of products. Linoleic Acid API Price is not always fixed or binding as the Linoleic Acid Price is obtained through a variety of data sources. The Linoleic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Linoleic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Linoleic Acid, including repackagers and relabelers. The FDA regulates Linoleic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Linoleic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Linoleic Acid supplier is an individual or a company that provides Linoleic Acid active pharmaceutical ingredient (API) or Linoleic Acid finished formulations upon request. The Linoleic Acid suppliers may include Linoleic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Linoleic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Linoleic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Linoleic Acid active pharmaceutical ingredient (API) in detail. Different forms of Linoleic Acid DMFs exist exist since differing nations have different regulations, such as Linoleic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Linoleic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Linoleic Acid USDMF includes data on Linoleic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Linoleic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Linoleic Acid suppliers with USDMF on PharmaCompass.
Linoleic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Linoleic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Linoleic Acid GMP manufacturer or Linoleic Acid GMP API supplier for your needs.
A Linoleic Acid CoA (Certificate of Analysis) is a formal document that attests to Linoleic Acid's compliance with Linoleic Acid specifications and serves as a tool for batch-level quality control.
Linoleic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Linoleic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Linoleic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Linoleic Acid EP), Linoleic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Linoleic Acid USP).

