
Synopsis
Synopsis
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
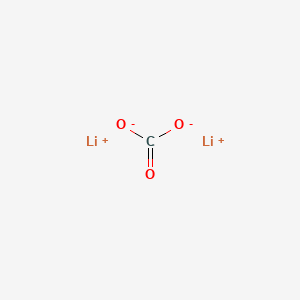

1. Bicarbonate, Lithium
2. Carbonate, Dilithium
3. Carbonate, Lithium
4. Cp 15,467 61
5. Cp-15,467-61
6. Cp15,46761
7. Dilithium Carbonate
8. Eskalith
9. Lithane
10. Lithium Bicarbonate
11. Lithobid
12. Lithonate
13. Lithotabs
14. Micalith
15. Nsc 16895
16. Nsc-16895
17. Nsc16895
18. Priadel
19. Quilinorm Retard
20. Quilinorm-retard
21. Quilinormretard
1. 554-13-2
2. Dilithium Carbonate
3. Lithonate
4. Lithobid
5. Lithane
6. Carbonic Acid, Dilithium Salt
7. Eskalith
8. Lithotabs
9. Carbonic Acid Lithium Salt
10. Liskonum
11. Lithizine
12. Micalith
13. Priadel
14. Limas
15. Eskalith Cr
16. Camcolit
17. Carbolitium
18. Neurolepsin
19. Lithium Carbonicum
20. Nsc-16895
21. Candamide
22. Carbolith
23. Eutimin
24. Hypnorex
25. Lithicarb
26. Lithinate
27. Lithionate
28. Liticar
29. Manialith
30. Maniprex
31. Litard
32. Lithea
33. Plenur
34. Quilonum Retard
35. Pfi-lithium
36. Lithium Phasal
37. Pfl-lithium
38. Litho-carb
39. Cp-15467-61
40. Lithium Carbonate (li2co3)
41. Cp-15,467-61
42. Mfcd00011084
43. 2bmd2gna4v
44. Chebi:6504
45. Carbonic Acid Lithium Salt (li2co3)
46. Lithium Carbonate (2:1)
47. Cp 15467-61
48. Ceglution
49. Phasal
50. Cp-1546761
51. Teralithe [french]
52. Lithium Carbonate Nanoparticles
53. Carbolithium
54. Teralithe
55. Lithium, Reference Standard Solution
56. Dilithium;carbonate
57. Li2 (c O3)
58. Carbonic Acid Dilithium Salt
59. Ccris 3153
60. Hsdb 3351
61. Einecs 209-062-5
62. Unii-2bmd2gna4v
63. Carbolithium Ifi
64. Lithium Qd
65. Eskalith (tn)
66. Lithobid (tn)
67. Lithium Carbonate [usan:usp:jan]
68. Starbld0023766
69. Lithium Carbonate Powder
70. Li2co3
71. Dilithium Trioxidocarbonate
72. Ec 209-062-5
73. Lithium Carbonate [mi]
74. Lithium Carbonate [jan]
75. Chembl1200826
76. Dtxsid1023784
77. Lithium Carbonate (jp17/usp)
78. Lithium Carbonate [hsdb]
79. Lithium Carbonate [inci]
80. Lithium Carbonate [usan]
81. Lithium Carbonate [vandf]
82. Lithium Carbonicum [hpus]
83. Lithium Carbonate [mart.]
84. Lithium Carbonate [usp-rs]
85. Lithium Carbonate [who-dd]
86. Lithium Carbonate [who-ip]
87. Str02638
88. Carbonic Acid, Lithium Salt (1:2)
89. Dilithium Carbonate [who-ip]
90. Lithium Carbonate, Acs Reagent Grade
91. Akos015904647
92. Angc-554-13-2
93. Db14509
94. Lithii Carbonas [who-ip Latin]
95. Lithium Carbonate [orange Book]
96. Lithium Carbonate [ep Monograph]
97. Lithium Carbonate [usp Monograph]
98. B7705
99. Ft-0627895
100. L0224
101. C07964
102. D00801
103. Dilithium Carbonate, Dilithium Salt, Carbonic Acid
104. Q410174
| Molecular Weight | 73.9 g/mol |
|---|---|
| Molecular Formula | CLi2O3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 74.01675073 g/mol |
| Monoisotopic Mass | 74.01675073 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Lithium carbonate |
| Drug Label | Each capsule for oral administration contains 150 mg, 300 mg or 600 mg of Lithium Carbonate USP.Inactive IngredientsThe capsules contain talc. The hard gelatin shell consists of gelatin, titanium dioxide, sodium lauryl sulphate and FD & C Red 40.The.. |
| Active Ingredient | Lithium carbonate |
| Dosage Form | Tablet, extended release; Tablet; Capsule |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg; 450mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Alembic; Hetero Labs Ltd Iii; Sun Pharm Inds; Hikma Pharms; Roxane; Glenmark Generics |
| 2 of 4 | |
|---|---|
| Drug Name | Lithobid |
| Drug Label | LITHOBID tablets contain lithium carbonate, a white odorless alkaline powder with molecular formula Li2CO3 and molecular weight 73.89. Lithium is an element of the alkali-metal group with atomic number 3, atomic weight 6.94, and an emission line at... |
| Active Ingredient | Lithium carbonate |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Ani Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Lithium carbonate |
| Drug Label | Each capsule for oral administration contains 150 mg, 300 mg or 600 mg of Lithium Carbonate USP.Inactive IngredientsThe capsules contain talc. The hard gelatin shell consists of gelatin, titanium dioxide, sodium lauryl sulphate and FD & C Red 40.The.. |
| Active Ingredient | Lithium carbonate |
| Dosage Form | Tablet, extended release; Tablet; Capsule |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg; 450mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Alembic; Hetero Labs Ltd Iii; Sun Pharm Inds; Hikma Pharms; Roxane; Glenmark Generics |
| 4 of 4 | |
|---|---|
| Drug Name | Lithobid |
| Drug Label | LITHOBID tablets contain lithium carbonate, a white odorless alkaline powder with molecular formula Li2CO3 and molecular weight 73.89. Lithium is an element of the alkali-metal group with atomic number 3, atomic weight 6.94, and an emission line at... |
| Active Ingredient | Lithium carbonate |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Ani Pharms |
Antidepressive Agents; Antimanic Agents; Antithyroid Agents; Enzyme Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Most prepn currently used in the U.S. are tablets or capsules of lithium carbonate. Slow-release prepn of lithium carbonate also are available, as is a liq prepn of lithium citrate (with 8 mEq of Li+, equivalent to 300 mg of carbonate salt, per 5 mL or 1 teaspoonful of citrate liq). Salts other than the carbonate have been used, but the carbonate salt is favored for tablets and capsules because it is relatively less hygroscopic and less irritating to the gut than other salts, especially the chloride salt. /Li therapy/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 511
Lithium /carbonate and citrate/ is indicated as the primary agent in the treatment of acute manic and hypomanic episodes in bipolar disorder, and for maintenance therapy to help diminish the intensity and frequency of subsequent manic episodes in patients with a history of mania. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1844
Lithium /carbonate and citrate/ is used in some patients as the agent of choice in the prevention of bipolar depression. Clinicians have observed a diminished intensity and frequency of severe depressive episodes. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1844
For more Therapeutic Uses (Complete) data for LITHIUM CARBONATE (28 total), please visit the HSDB record page.
Because of potential effects of lithium upon thyroid and renal function, appropriate indices of these functions should be measured prior to start of treatment, then monitored periodically as therapy proceeds.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 580
Lithium carbonate should not be used in patients with cardiovascular or renal disease. ...Should not be used in children under 12 yr of age.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1025
Outpatients and their families should be warned that the patient must discontinue lithium therapy immediately and consult a physician if signs of lithium intoxication such as muscle twitching, tremor, mild ataxia, drowsiness, muscle weakness, diarrhea, or vomiting occur. Patients also should be warned that lithium may impair their ability to perform activities requiring mental alertness or physical coordination (e.g., operating machinery, driving a motor vehicle).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2570
Lithium should be used cautiously in patients with preexisting cardiovascular or thyroid disease. Patients with underlying cardiovascular disease should be observed carefully for signs and symptoms of arrhythmia (including periodic ECG determinations), and serum lithium concentrations should be kept within the therapeutic range since nodal arrhythmias may occur. Patients with underlying hypothyroidism should have thyroid function (T3, T4, and TSH concentrations) evaluated yearly and be given supplemental thyroid therapy when needed.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2571
For more Drug Warnings (Complete) data for LITHIUM CARBONATE (45 total), please visit the HSDB record page.
Lithium is used as a mood stabilizer, and is indicated for the treatment of manic episodes and maintenance of bipolar disorder.
FDA Label
Lithium's mechanism of action is still unknown. Lithium's therapeutic action may be due to a number of effects, ranging from inhibition of enzymes such as glycogen synthase kinase 3, inositol phosphatases, or modulation of glutamate receptors.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antimanic Agents
Agents that are used to treat bipolar disorders or mania associated with other affective disorders. (See all compounds classified as Antimanic Agents.)
Absorption
Lithium absorption is rapid and oral bioavailability is close to 100%.
Route of Elimination
Lithium is primarily eliminated through the kidneys and elimination in the feces is insignificant.
Volume of Distribution
Apparent volume of distribution is 0.7 to 1.0L/kg.
Clearance
Clearance is generally between 10 and 40mL/min but may be as low as 15mL/min in elderly patients and those with renal impairment.
... Lithium /carbonate/ crosses placenta and is present in mother and fetus in same concentration.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 313
Lithium /carbonate/ is completely absorbed six to eight hours after oral administration. Since the onset of action is slow (five to ten days), parenteral administration is of no advantage. The plasma half-life is 17 to 36 hours, and this drug is eliminated almost entirely by the kidneys. Lithium clearance averages approximately 20% of creatinine clearance, but significant variability exists among patients.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 313
The population pharmacokinetics of lithium were determined using the nonlinear mixed effects model (NONMEM) program in 79 psychiatric inpatients who were at least 18 yr old, had normal renal function, and were receiving lithium carbonate 2 or 3 times daily. With the initial model, the mean lithium volume of distribution was 32.8 L and the mean lithium clearance was 1.36 L/hr. With an intermediate model, lithium clearance estimates improved on the basis of patient size (weight and body surface area), daily lithium dosage, age, gender, and race. When only the most significant variables, lean body weight and creatinine clearance, were retained, a final model was obtained that yielded a coefficient of variation for lithium clearance of about 24% and gave fairly accurate predictions of steady-state lithium concentrations (coefficient of variation, about 16%). It was concluded that analysis of lithium pharmacokinetics with the nonlinear mixed-effects model program suggested that lean body weight and creatinine clearance are important predictors of lithium clearance.
PMID:2049899 Jermain DE et al; Clin Pharm 10 (May): 376-381 (1991)
Lithium disposition in plasma, red blood cells and urine was studied in acute self-poisoned patient upon chronic lithium therapy (n=4) and in chronic intoxicated patients receiving oral lithium (n=10). Following acute intoxication upon chronic lithium therapy, lithium pharmacokinetics did not differ from previous reports. Terminal plasma half life ranged from 19.0-29.0 hr and RBC/plasma ratio was 0.32 +/- 0.11. the distribution volume of the terminal phase, Vz, was estimated at 0.84 +/- 0.32 L/kg and renal clearance was 0.38 +/- 0.11 ml/mn/kg. After chronic intoxication lithium pharmacokinetics differed from those of the acute patients. Terminal plasma half-life ranged from 36.5-79.4 hr and zero-order decline appeared in 8 of the 10 patients. The RBC/plasma ratio was 0.87 +/- 0.22 on admission. Vz was estimated at 0.71 +/- 0.27 L/kg and renal clearance was 0.16 +/- 0.07 ml/mn/kg. These modifications in lithium elimination kinetics could be related to the decrease in the glomerular filtration rate with age or renal dysfunction in this group of patients.
PMID:7582387 Ferron G et al; Int J Clin Pharmacol Ther 33 (6): 351-5 (1995)
For more Absorption, Distribution and Excretion (Complete) data for LITHIUM CARBONATE (22 total), please visit the HSDB record page.
Lithium carbonate is not metabolized before excretion.
The half life of lithium carbonate is 18 to 36 hours. Other sources say it may be 7 to 20 hours.
The plasma half-life is 17 to 36 hours.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 313
The plasma half-life (in healthy volunteers) shows a considerable variability: from 5 to 40 hr, with most values between 15 and 30 hr, it depends on the duration of treatment as well as on kidney function and age. /Li+/
Chang, L.W. (ed.). Toxicology of Metals. Boca Raton, FL: Lewis Publishers, 1996, p. 455
The usual elimination half-life is 12 to 27 hr, but it may rise to nearly 60 hr if renal excretion is compromised. /Li+/
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 852
... The elimination half-life averages 20 to 24 hr. /Li+/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 508
For more Biological Half-Life (Complete) data for LITHIUM CARBONATE (9 total), please visit the HSDB record page.
Lithium's mechanism of action is still unknown. However, the inositol depletion theory suggests 3 main potential targets. These targets are inositol monophosphatase, inositol polyphosphatase, and glycogen synthase kinase 3(GSK-3). The Inositol depletion theory suggests lithium behaves as an uncompetitive inhibitor of inositol monophosphatase in a manner inversely proportional to the degree of stimulus. This inhibition lowers levels of inositol triphosphate. However, stronger inhibitors of inositol monophosphatase are not as clinically effective and low levels of inositol triphosphate are associated with memory impairment. Lithium acts on inositol polyphosphatase as an uncompetitive inhibitor. This inhibition is thought to have multiple downstream effects that have yet to be clarified. Lithium regulates phosphorylation of GSK-3 which regulates other enzymes through phosphorylation. Lithium can also inhibit GSK-3 through interfering with the magnesium ion in the active site.
Although its antimanic mechanism of action has not been fully determined, lithium is known to affect a variety of neurohumoral signal transduction mechanisms. ... Lithium /lithium carbonate/ counteracts mood changes and is considered the most specific antimanic drug for the prophylaxis and treatment of bipolar disorder.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 311
In animal brain tissue, Li+ at concn of 1 to 10 mEq/L inhibits the depolarization-provoked and Ca+2-dependent release of norepinephrine and dopamine, but not serotonin, from nerve terminals. Li+ may even enhance the release of serotonin, especially in the limbic system, at least transiently. The ion has little effect on catecholamine-sensitive adenylyl cyclase activity or on the binding of ligands to monoamine receptors in brain tissue, although there is some evidence that Li+ can inhibit the effects of receptor-blocking agents that cause supersensitivity in such systems. Li+ can modify some hormonal responses mediated by adenylyl cyclase or phospholipase C in other tissues, including the actions of antidiuretic and thyroid-stimulating hormones on the actions of antidiuretic and thyroid-stimulating hormones on their peripheral target tissues. In part, the actions of Li+ may reflect its ability to interfere with the activity of both stimulatory and inhibitory GTP-binding proteins (Gs and Gi) by keeping them in their less active alpha-beta-gamma trimer state. /Li+/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 508
Lithium inhibits the phosphatase that liberates inositol (I) from inositol phosphate (IP) ... Li+ ... can modify the abundance or function of G proteins and effectors, as well as protein kinases and several cell and nuclear regulatory factors. /Li+, from figure/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 494
Mechanistically, the Li effect results from its substitution for body cations, eg, sodium and potassium, resulting in multisystemic actions. A partial substitution for normal cations causes changes in ion exchange and transfer in cellular processes. Incorporation of Li into membrane structures may alter responses to hormones and the coupling of energy processes ... Among the factors that may modify Li toxicity and kinetics are the type of the poisoning, the presence of the underlying disease, and renal impairment. /Li+/
Chang, L.W. (ed.). Toxicology of Metals. Boca Raton, FL: Lewis Publishers, 1996, p. 455
For more Mechanism of Action (Complete) data for LITHIUM CARBONATE (22 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : LITHIUM CARBONATE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG
Approval Date : 2009-01-12
Application Number : 79159
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : LITHIUM CARBONATE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 600MG
Approval Date : 2009-01-12
Application Number : 79159
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
Brand Name : LITHOBID
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 300MG
Approval Date : 1982-01-01
Application Number : 18027
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LITHIUM CARBONATE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 300MG
Approval Date : 2002-06-10
Application Number : 76170
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : LITHIUM CARBONATE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 300MG
Approval Date : 2004-10-28
Application Number : 76832
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ESKALITH
Dosage Form : TABLET;ORAL
Dosage Strength : 300MG
Approval Date : 1982-01-01
Application Number : 17971
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LITHONATE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG
Approval Date : 1982-01-01
Application Number : 16782
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LITHIUM CARBONATE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 600MG
Approval Date : 2004-06-29
Application Number : 76823
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Brand Name : LITHIUM CARBONATE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG
Approval Date : 2002-06-27
Application Number : 76243
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Brand Name : LITHIUM CARBONATE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 450MG
Approval Date : 2012-08-08
Application Number : 202219
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Israel
Brand Name : Carbolithium
Dosage Form : Lithium Carbonate 150Mg 50 Combined Oral Use
Dosage Strength : 50 cps 150 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Israel

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Priadel retard
Dosage Form : Tablet
Dosage Strength : 400mg
Packaging :
Approval Date : 13/08/1970
Application Number : 35380
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Plenur
Dosage Form : Modified Release Tablet
Dosage Strength : 400MG
Packaging :
Approval Date : 01-03-1970
Application Number : 48932
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : United Kingdom
Brand Name : Lithium Carbonate
Dosage Form :
Dosage Strength : 50 Cpr 300 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : United Kingdom

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Lithiofor
Dosage Form : Modified Release Tablet
Dosage Strength : 660mg
Packaging :
Approval Date : 17/08/1973
Application Number : 38061
Regulatory Info : Allowed
Registration Country : Switzerland

Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : LiDCO Lithiumchlorid mmolml
Dosage Form : Solution For Injection
Dosage Strength : 0.15mmol/ml
Packaging :
Approval Date : 28/02/2007
Application Number : 57447
Regulatory Info : Allowed
Registration Country : Switzerland

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Camcolit
Dosage Form : Film Coated Tablet
Dosage Strength : 400MG
Packaging :
Approval Date : 2013-10-22
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Camcolit
Dosage Form : Film Coated Tablet
Dosage Strength : 400MG
Packaging :
Approval Date : 2020-11-19
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Carblite
Dosage Form : Tablet
Dosage Strength : 300mg
Packaging :
Approval Date : 15-01-2025
Application Number : 28106969723
Regulatory Info : Prescription
Registration Country : Denmark

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Litiumkarbonat \"Oba\"
Dosage Form : Film Coated Tablet
Dosage Strength : 300mg
Packaging :
Approval Date : 14-12-1976
Application Number : 28100731076
Regulatory Info : Prescription
Registration Country : Denmark

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
59
PharmaCompass offers a list of Lithium Carbonate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lithium Carbonate manufacturer or Lithium Carbonate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lithium Carbonate manufacturer or Lithium Carbonate supplier.
PharmaCompass also assists you with knowing the Lithium Carbonate API Price utilized in the formulation of products. Lithium Carbonate API Price is not always fixed or binding as the Lithium Carbonate Price is obtained through a variety of data sources. The Lithium Carbonate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lithium Carbonate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lithium Carbonate, including repackagers and relabelers. The FDA regulates Lithium Carbonate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lithium Carbonate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lithium Carbonate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lithium Carbonate supplier is an individual or a company that provides Lithium Carbonate active pharmaceutical ingredient (API) or Lithium Carbonate finished formulations upon request. The Lithium Carbonate suppliers may include Lithium Carbonate API manufacturers, exporters, distributors and traders.
click here to find a list of Lithium Carbonate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lithium Carbonate DMF (Drug Master File) is a document detailing the whole manufacturing process of Lithium Carbonate active pharmaceutical ingredient (API) in detail. Different forms of Lithium Carbonate DMFs exist exist since differing nations have different regulations, such as Lithium Carbonate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lithium Carbonate DMF submitted to regulatory agencies in the US is known as a USDMF. Lithium Carbonate USDMF includes data on Lithium Carbonate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lithium Carbonate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lithium Carbonate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lithium Carbonate Drug Master File in Japan (Lithium Carbonate JDMF) empowers Lithium Carbonate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lithium Carbonate JDMF during the approval evaluation for pharmaceutical products. At the time of Lithium Carbonate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lithium Carbonate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lithium Carbonate Drug Master File in Korea (Lithium Carbonate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lithium Carbonate. The MFDS reviews the Lithium Carbonate KDMF as part of the drug registration process and uses the information provided in the Lithium Carbonate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lithium Carbonate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lithium Carbonate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lithium Carbonate suppliers with KDMF on PharmaCompass.
A Lithium Carbonate CEP of the European Pharmacopoeia monograph is often referred to as a Lithium Carbonate Certificate of Suitability (COS). The purpose of a Lithium Carbonate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lithium Carbonate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lithium Carbonate to their clients by showing that a Lithium Carbonate CEP has been issued for it. The manufacturer submits a Lithium Carbonate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lithium Carbonate CEP holder for the record. Additionally, the data presented in the Lithium Carbonate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lithium Carbonate DMF.
A Lithium Carbonate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lithium Carbonate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lithium Carbonate suppliers with CEP (COS) on PharmaCompass.
A Lithium Carbonate written confirmation (Lithium Carbonate WC) is an official document issued by a regulatory agency to a Lithium Carbonate manufacturer, verifying that the manufacturing facility of a Lithium Carbonate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lithium Carbonate APIs or Lithium Carbonate finished pharmaceutical products to another nation, regulatory agencies frequently require a Lithium Carbonate WC (written confirmation) as part of the regulatory process.
click here to find a list of Lithium Carbonate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lithium Carbonate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lithium Carbonate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lithium Carbonate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lithium Carbonate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lithium Carbonate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lithium Carbonate suppliers with NDC on PharmaCompass.
Lithium Carbonate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lithium Carbonate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lithium Carbonate GMP manufacturer or Lithium Carbonate GMP API supplier for your needs.
A Lithium Carbonate CoA (Certificate of Analysis) is a formal document that attests to Lithium Carbonate's compliance with Lithium Carbonate specifications and serves as a tool for batch-level quality control.
Lithium Carbonate CoA mostly includes findings from lab analyses of a specific batch. For each Lithium Carbonate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lithium Carbonate may be tested according to a variety of international standards, such as European Pharmacopoeia (Lithium Carbonate EP), Lithium Carbonate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lithium Carbonate USP).

