
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
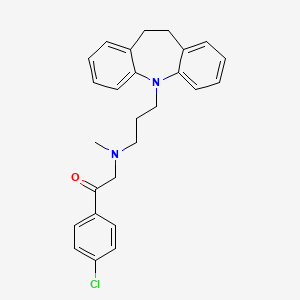

1. Deftan
2. Feprapax
3. Gamanil
4. Gamonil
5. Hydrochloride, Lofepramine
6. Leo 640
7. Lofepramine Hydrochloride
8. Lomont
9. Lopramine
1. Lopramine
2. 23047-25-8
3. Leo 640
4. Amplit
5. Gamanil
6. 4'-chlor-2-((3-(10,11-dihydro-5h-dibenz(b,f)azepin-5-yl)propyl)methylamino)acetophenon
7. Oca4jt7paw
8. Ethanone, 1-(4-chlorophenyl)-2-((3-(10,11-dihydro-5h-dibenz(b,f)azepin-5-yl)propyl)methylamino)-
9. Chebi:47782
10. Lofepramine (inn)
11. 1-(4-chlorophenyl)-2-{[3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)propyl](methyl)amino}ethanone
12. Lofepramine [inn]
13. 1-(4-chlorophenyl)-2-[3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)propyl-methylamino]ethanone
14. Acetophenone, 4'-chloro-2-((3-(10,11-dihydro-5h-dibenz(b,f)azepin5-yl)propyl)methylamino)-
15. 1-(4-chlorophenyl)-2-((3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)propyl)(methyl)amino)ethan-1-one
16. Lofepramina
17. Lofepraminum
18. Lomont
19. Lofepramine [inn:ban]
20. Lofepraminum [inn-latin]
21. Lofepramina [inn-spanish]
22. 4'-chloro-2-((3-(10,11-dihydro-5h-dibenz(b,f)azepin5-yl)propyl)methylamino)acetophenone
23. 4'-chloro-2-[[3-(10,11-dihydro-5h-dibenz[b,f]azepin-5-yl)propyl]methylamino]acetophenone
24. 1-(4-chlorophenyl)-2-{[3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)propyl]methylamino}ethanone
25. Ethanone, 1-(4-chlorophenyl)-2-[[3-(10,11-dihydro-5h-dibenz[b,f]azepin-5-yl)propyl]methylamino]-
26. Hsdb 7184
27. Unii-oca4jt7paw
28. Ncgc00166397-02
29. Einecs 245-396-8
30. Db 2182
31. 4'-chloro-2-((3-(10,11-dihydro-5h-dibenz(b,f)azepin-5-yl)propyl)methylamino)acetophenone
32. Lofepramine- Bio-x
33. Gamanil (salt/mix)
34. Gamonil (salt/mix)
35. Timelit (salt/mix)
36. Tymelyt (salt/mix)
37. Lofepramine [mi]
38. Lofepramine [jan]
39. Lofepramine [hsdb]
40. Schembl35028
41. Lofepramine [who-dd]
42. Chembl87708
43. Gtpl7551
44. Dtxsid2023220
45. Bdbm82437
46. Hms3269o19
47. Hms3413f21
48. Hms3677f21
49. Hms3715l16
50. Nsc_3947
51. Zinc1542929
52. Lofepramin Hydrochloride (salt/mix)
53. Akos024457158
54. Ccg-221257
55. Db13411
56. Ncgc00166397-01
57. Ncgc00166397-03
58. Bl164630
59. Hy-12390
60. Us8629135, Sw-06
61. Cas_23047-25-8
62. B7097
63. Cs-0011244
64. Ft-0670832
65. D08140
66. Ab00698521-05
67. A854424
68. L000944
69. Q368941
70. J-014957
71. 1-(4-chlorophenyl)-2-[[3-(10,11-dihydro-5h-dibenz(z)[b,f]azepin-5-yl)propyl]methylamino]ethanone
72. 1-(4-chlorophenyl)-2-[[3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)propyl](methyl)amino]ethanone #
73. N-methyl-n(4-chlorobenzoylmethyl)-3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)propylamine Hydrochloride
| Molecular Weight | 419.0 g/mol |
|---|---|
| Molecular Formula | C26H27ClN2O |
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 418.1811912 g/mol |
| Monoisotopic Mass | 418.1811912 g/mol |
| Topological Polar Surface Area | 23.6 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 523 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Lofepramine is a tricyclic antidepressant related to imipramine. Meta-analyses were carried out with respect to efficacy and tolerability by combining outcome and adverse reaction from over 20 controlled trials comparing lofepramine with other tricyclic antidepressants. Lofepramine was at least as effective as the comparators with fewer adverse effects. In particular, the risk/benefit ratio seemed superior to the comparators amitriptyline, imipramine, clomipramine, maprotiline and desipramine.
PMID:1834491 Kerihuel JC, Dreyfus JF; J Int Med Res 19 (3): 183-201 (1991)
The results of double blind trial in which 139 patients with primary depression were randomly assigned to either lofepramine (46), imipramine (48), or placebo (45) are discussed. After treatment with either active drug, lofepramine or imipramine, the clinical outcome was significantly greater than with placebo. No significant differences were found in clinical responses between lofepramine and imipramine. With regard to reported side effects, however, a statistically significant lower number of severe and/or moderate side effects were reported for the lofepramine group than for the imipramine group.
PMID:6753500 Feighner JP et al; Acta Psychiatr Scand 66 (2): 100-8 (1982)
EXPTL Therapy: In a randomized, placebo-controlled double-blind trial a combination of lofepramine, phenylalanine and vitamin B(12) was found to be effective in relieving the symptoms of multiple sclerosis (MS). The effect occurred within 2-4 weeks, and improved all types of symptoms in all types of MS. The combination was also effective in relieving symptoms in patients with chronic pain and chronic fatigue.
PMID:12376086 Loder C et al; Med Hypotheses 59 (5): 594-602 (2002)
EXPTL Therapy: A double-blind randomised controlled trial of the effect of low dose lofepramine (70 mg once daily) against placebo was carried out on depressed elderly inpatients on general medical wards for the elderly, comparing measures of depression and side-effects between the randomised groups. Patients were identified for the study using the Geriatric Depression Scale (GDS) and the Brief Assessment Schedule Depression Cards (BASDEC). Sixty-three subjects were randomised: 46 patients completed the entire trial of 28 days treatment. BASDEC and GDS were administered on day 8 post-admission, and depressed patients were randomised double-blind to either low dose lofepramine (70 mg daily) (n = 23) or placebo (n = 23). Assessment of changes in depressive states were made using the Montgomery Asberg Depression Rating Scale (MADRS) on days 8, 18 and 36 post-admission. Both groups improved by a similar amount during the trial. Lofepramine tended to be more effective than placebo in those patients who were more depressed (GDS > or = 18). On the other hand, subjects who were less depressed (i.e. GDS < 18) improved more on placebo than lofepramine. Low dose lofepramine may prove useful in moderately or severely depressed patients treated for only 4 weeks. However, low dose lofepramine is not indicated for mild (GDS 15-18) depression.
PMID:8018452 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1364731 Tan RS et al; Br J Clin Pharmacol 37 (4): 321-4 (1994)
Dry mouth is the most common side effect. Constipation, dizziness, sweating, nausea/vomiting, tremor palpitation, blurred vision, drowsiness, fatigue, headache, and insomnia were also observed during clinical studies. There does not appear to be a correlation between plasma lofepramine levels and adverse effects.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 646
Lofepramine is a tricyclic antidepressant that is structurally similar to imipramine ... . Dry mouth is the most commonly reported side effect of usual therapeutic doses of lofepramine, but the incidence of this and other anticholinergic side effects is less among patients treated with lofepramine than with imipramine. Lofepramine has not been associated with adverse effects on cardiac function even in cases of attempted suicide by overdose.
Lancaster SG, Gonzalez JP; Drugs 37 (2): 123-40 (1989)
Physostigmine should not be used as an antidote for cyclic antidepressant overdose because it may worsen cardiac conduction disturbances, cause bradyarrhythmias or asystole, and aggravate or precipitate seizures. /Physostigmine/
Olson, K.R. (Ed.); Poisoning & Drug Overdose. 4th ed. Lange Medical Books/McGraw-Hill. New York, N.Y. 2004., p. 490
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AA - Non-selective monoamine reuptake inhibitors
N06AA07 - Lofepramine
Following oral administration of a single dose of 210 mg to healthy subjects, lofepramine is rapidly absorbed, with peak plasma concentrations of 140 ng/ml(lofepramine) and 5 ng/ml (desipramine) achieved within 1 and 4 hours, respectively.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 645
The in vitro metabolism of lofepramine was studied in comparison with imipramine. Both compounds were hydroxylated and demethylated by a NADPH-generating system in rat and human liver microsomes. Three metabolites were in common for the two drugs, namely desipramine (DMI), 2-hydroxydesipramine (2-OH-DMI) and didesmethylimipramine (DDMI). Lofepramine was also metabolized to three unique tricyclic metabolites. Comparisons with authentic reference compounds suggested that two of these metabolites were 2-hydroxylofepramine and desmethyllofepramine. The ratio between the concentrations of DDMI and DMI was higher for lofepramine than imipramine. This is probably due to DDMI formation via two parallel metabolic pathways of lofepramine, i.e. DMI and desmethyllofepramine, respectively. It is speculated that the different metabolic pattern of lofepramine as compared with desipramine and imipramine is of importance for the therapeutic profile of the drug.
PMID:7839694 Strandgarden K, Gunnarsson PO; Xenobiotica 24 (8): 703-11 (1994)
Lofepramine is partially metabolized to desipramine by a cytochrome p450 dependent enzyme system on first pass through the liver. It is metabolized by N-dealkylation, hydroxylation, and glucuronidation. The plasma clearance of lofepramine is 686 L/hr. Its main urinary metabolites are desipramine, 2-hydroxydesipramine, and its glucuronide (2-hydroxyimodibenzyl), and the glycine conjugate of p-chlorobenzoic acid.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 645
The mean elimination half-lives are 1.7 hours for a 70 mg dose and 2.5 hours for a 140 mg dose. Lofepramine has a half-life in the beta phase of about 5 hours (compared with 24 hours for other TCAs).
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 645
Lofepramine is a tricyclic antidepressant that is structurally similar to imipramine and is extensively metabolised to desipramine. In the absence of other major pharmacological effects it appears that its antidepressant activity stems from the facilitation of noradrenergic neurotransmission by uptake inhibition, and possibly by the additional facilitation of serotoninergic neurotransmission.
Lancaster SG, Gonzalez JP; Drugs 37 (2): 123-40 (1989)
ABOUT THIS PAGE
29
PharmaCompass offers a list of Lofepramine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lofepramine manufacturer or Lofepramine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lofepramine manufacturer or Lofepramine supplier.
PharmaCompass also assists you with knowing the Lofepramine API Price utilized in the formulation of products. Lofepramine API Price is not always fixed or binding as the Lofepramine Price is obtained through a variety of data sources. The Lofepramine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lofepramine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lofepramine, including repackagers and relabelers. The FDA regulates Lofepramine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lofepramine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Lofepramine supplier is an individual or a company that provides Lofepramine active pharmaceutical ingredient (API) or Lofepramine finished formulations upon request. The Lofepramine suppliers may include Lofepramine API manufacturers, exporters, distributors and traders.
Lofepramine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lofepramine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lofepramine GMP manufacturer or Lofepramine GMP API supplier for your needs.
A Lofepramine CoA (Certificate of Analysis) is a formal document that attests to Lofepramine's compliance with Lofepramine specifications and serves as a tool for batch-level quality control.
Lofepramine CoA mostly includes findings from lab analyses of a specific batch. For each Lofepramine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lofepramine may be tested according to a variety of international standards, such as European Pharmacopoeia (Lofepramine EP), Lofepramine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lofepramine USP).
