
Synopsis
Synopsis
0
JDMF
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
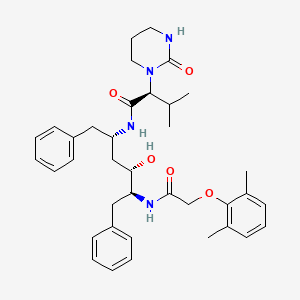

1. A 157378.0
2. A-157378.0
3. A157378.0
4. Abt 378
5. Abt-378
6. Abt378
7. N-(4-(((2,6-dimethylphenoxy)acetyl)amino)-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2h)-pydrimidineacetamide
1. 192725-17-0
2. Abt-378
3. Aluviran
4. Koletra
5. Abt 378
6. A 157378.0
7. A-157378.0
8. Lopinavir-
9. Rs-346
10. Chebi:31781
11. A-157378-0
12. Lopinavir (abt-378)
13. (alphas)-tetrahydro-n-((alphas)-alpha-((2s,3s)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-alpha-isopropyl-2-oxo-1(2h)-pyrimidineacetamide
14. Lpv
15. Lopinavir, (s-(2s,4s,5s))-
16. A 157378
17. 2494g1jf75
18. (2s)-n-[(2s,4s,5s)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
19. (s)-n-((2s,4s,5s)-5-(2-(2,6-dimethylphenoxy)acetamido)-4-hydroxy-1,6-diphenylhexan-2-yl)-3-methyl-2-(2-oxotetrahydropyrimidin-1(2h)-yl)butanamide
20. N-{1-benzyl-4-[2-(2,6-dimethyl-phenoxy)-acetylamino]-3-hydroxy-5-phenyl-pentyl}-3-methyl-2-(2-oxo-tetrahydro-pyrimidin-1-yl)-butyramide
21. Ncgc00164576-02
22. Dsstox_cid_26456
23. Dsstox_rid_81630
24. Dsstox_gsid_46456
25. (2s)-n-[(2s,4s,5s)-5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
26. (2s)-n-[(1s,3s,4s)-1-benzyl-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-pentyl]-3-methyl-2-(2-oxohexahydropyrimidin-1-yl)butanamide
27. Ab1
28. Smr002529581
29. Cas-192725-17-0
30. Lopinavirum
31. Abt378
32. Unii-2494g1jf75
33. Hsdb 8138
34. 1mui
35. 2qhc
36. 2rkf
37. 2rkg
38. 3ogq
39. Lopinavir [usan:usp:inn:ban]
40. Aids032937
41. Lopinavir, Bio-x
42. (2s)-n-((1s,3s,4s)-1-benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenylpentyl)-3-methyl-2-(2-oxotetrahydropyrimidin-1(2h)-yl)butanamide
43. (2s)-n-[(1s,3s,4s)-1-benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenylpentyl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2h)-yl)butanamide
44. Lopinavir & Plga
45. Kaletra (lpv+rtv)
46. 2o4s
47. 2q5k
48. 4l1a
49. Lopinavir [inn]
50. Lopinavir [jan]
51. Lopinavir [mi]
52. Lopinavir [usan]
53. Lopinavir [vandf]
54. Chembl729
55. Lopinavir [mart.]
56. Lpv & Aag
57. Lopinavir [usp-rs]
58. Lopinavir [who-dd]
59. Lopinavir [who-ip]
60. Lopinavir (jan/usp/inn)
61. Schembl21775
62. Lopinavir [ema Epar]
63. (1s-(1r*(r*),3r*,4r*))-n-(4-(((2,6-dimethylphenoxy)acetyl)amino)-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2h)-pyrimidineacetamide
64. Mls003915624
65. Mls004774152
66. Mls006011206
67. Abt-378; Lopinavir
68. Lopinavir [orange Book]
69. Dtxsid8046456
70. Lopinavir [ep Monograph]
71. Gtpl11504
72. Lopinavir [usp Monograph]
73. Kaletra Component Lopinavir
74. Bcpp000184
75. Lopinavirum [who-ip Latin]
76. Ex-a4008
77. Zinc3951740
78. Tox21_112204
79. Bdbm50180655
80. Lopinavir & Alpha1-acid Glycoprotein
81. Mfcd22628840
82. S1380
83. Lopinavir Component Of Kaletra
84. Akos025243115
85. Lopinavir & Poly-lactide-co-glycolide
86. Tox21_112204_1
87. Bcp9000857
88. Ccg-270285
89. Cs-2077
90. Db01601
91. Ks-1436
92. Lopinavir 100 Microg/ml In Acetonitrile
93. Ncgc00164576-01
94. Ncgc00164576-03
95. Ncgc00164576-04
96. (2s)-n-[(2r,4s,5s)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenyl-hexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
97. (alphas)-n-[(1s,3s,4s)-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2h)-pyrimidineacetamide
98. 1(2h)-pyrimidineacetamide, N-((1s,3s,4s)-4-(((2,6-dimethylphenoxy)acetyl)amino)-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahyrdo-alpha-1-methylethyl)-2-oxo-, (alphas)-
99. Bl164636
100. Hy-14588
101. L0377
102. Sw219767-1
103. D01425
104. Ab01274785-01
105. Ab01274785_02
106. A813594
107. Q422585
108. Sr-01000931910
109. J-521653
110. Sr-01000931910-2
111. A-1573780
112. Brd-k99451608-001-02-4
113. (.alpha.s)-tetrahydro-n-((.alpha.s)-.alpha.-((2s,3s)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-.alpha.-isopropyl-2-oxo-1(2h)-pyrimidineacetamide
114. (1s-(1r*(r*),3r*,4r*))-n-(4-(((2,6-dimethylphenoxy)acetyl)amino)-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahydro-.alpha.-(1-methylethyl)-2-oxo-1(2h)-pyrimidineacetamide
115. (2s)-n-[(2s,4s,5s)-5-{[(2,6-dimethylphenoxy)acetyl]amino}-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2h)-yl)butanamide
116. 1(2h)-pyrimidineacetamide, N-[(1s,3s,4s)-4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-.alpha.-(1-methylethyl)-2-oxo-, (as)-
117. 1(2h)-pyrimidineacetamide, N-[(1s,3s,4s)-4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-, (alphas)- (9ci)
118. 1(2h)-pyrimidineacetamide, N-[(1s,3s,4s)-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-, (alphas)-
119. 1(2h)-pyrimidineacetamide, N-[4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-, [1s-[1r*(r*),3r*,4r*]]-
| Molecular Weight | 628.8 g/mol |
|---|---|
| Molecular Formula | C37H48N4O5 |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 15 |
| Exact Mass | 628.36247064 g/mol |
| Monoisotopic Mass | 628.36247064 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 940 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-HIV Agents; HIV Protease Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2013)
The fixed combination of lopinavir and ritonavir (lopinavir/ritonavir) is used in conjunction with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults, adolescents, and pediatric patients 14 days of age and older. Lopinavir/ritonavir is used in patients who are antiretroviral naive (have not previously received antiretroviral therapy) or antiretroviral experienced (received prior antiretroviral therapy). /Included in US product label/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 633
The manufacturer advises that the following factors be considered when initiating lopinavir/ritonavir. Administration of lopinavir/ritonavir in conjunction with other active antiretroviral agents is associated with a greater likelihood of treatment response. Use of lopinavir/ritonavir should be guided by results of genotypic or phenotypic viral resistance testing and/or the individual's prior antiretroviral treatment. The number of lopinavir resistance-associated mutations at baseline affects virologic response to lopinavir/ritonavir. Once-daily administration of lopinavir/ritonavir is not recommended in adults infected with HIV-1 strains with 3 or more viral mutations associated with lopinavir resistance and is not recommended in pediatric patients.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 633
Lopinavir/ritonavir is used in conjunction with other antiretrovirals for postexposure prophylaxis of HIV infection in individuals who have had nonoccupational exposure to blood, genital secretions, or other potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. /NOT included in US product label/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 634
For more Therapeutic Uses (Complete) data for Lopinavir (6 total), please visit the HSDB record page.
Serious and/or life-threatening adverse effects, clinically important drug interactions, or loss of virologic effect can occur if the fixed combination of lopinavir and ritonavir (lopinavir/ritonavir) is used concomitantly with some drugs. The potential for drug interactions must be considered prior to and during lopinavir/ritonavir therapy. Clinicians should review all drugs the patient is receiving and should monitor for adverse effects during lopinavir/ritonavir therapy.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 635
Lopinavir/ritonavir oral solution contains 42.4% (v/v) alcohol and 15.3% (w/v) propylene glycol. When administered concomitantly with propylene glycol, ethanol competitively inhibits metabolism of propylene glycol, which may lead to elevated propylene glycol concentrations. Preterm neonates /NOT included in US product label/may be at increased risk of propylene glycol-associated adverse effects due to diminished ability to metabolize propylene glycol, thereby leading to accumulation and potential adverse events. Life-threatening cardiac toxicity (including complete atrioventricular [AV] block, bradycardia, cardiomyopathy), lactic acidosis, acute renal failure, CNS depression, and respiratory complications leading to death have been reported, predominantly in preterm neonates /NOT included in US product label/ receiving lopinavir/ritonavir oral solution. Neonates, especially those born prematurely, are at risk of lopinavir, ethanol, and/or propylene glycol toxicity if they receive lopinavir/ritonavir oral solution.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 635
Once-daily regimens of lopinavir/ritonavir have not been evaluated in pediatric patients, and are not recommended in patients younger than 18 years of age.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 636
A safe and effective dose of lopinavir/ritonavir oral solution has not been established in neonates younger than 14 days of age /NOT included in US product label/ (whether born prematurely or full term). If the benefits of the oral solution for the treatment of HIV infection in an infant immediately after birth are judged to outweigh potential risks, the infant should be monitored closely for increases in serum osmolality and serum creatinine and other signs of toxicity related to the oral solution. These toxicities include hyperosmolality with or without lactic acidosis, renal toxicity, CNS depression (including stupor, coma, apnea), seizures, hypotonia, cardiac arrhythmias, ECG changes, and hemolysis. If lopinavir/ritonavir oral solution is used in preterm neonates /NOT included in US product label/ or in pediatric patients 14 days to 6 months of age, total amounts of alcohol and propylene glycol from all drugs that the child is receiving should be taken into account to avoid toxicity associated with these excipients.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 636
For more Drug Warnings (Complete) data for Lopinavir (19 total), please visit the HSDB record page.
The combination product lopinavir/ritonavir, marketed under the brand name Kaletra, is indicated in combination with other antiretrovirals for the treatment of HIV-1 infection in adults and pediatric patients 14 days old.
Lopinavir inhibits the activity of an enzyme critical for the HIV viral lifecycle. It has a moderate duration of action necessitating once or twice daily dosing. Lopinavir, like other protease inhibitors, has a propensity for participating in drug interactions - use caution when administering lopinavir to patients maintained on other pharmaceutical agents as pharmacodynamic and pharmacokinetic interactions are common. Fatal hepatotoxicity and pancreatitis have been noted in patients undergoing therapy with lopinavir and patients with an increased baseline risk of these events should be monitored closely throughout therapy.
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
J05AR10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Absorption
When administered alone, lopinavir has exceptionally low oral bioavailability (~25%) - for this reason, it is exclusively co-administered with ritonavir, which dramatically improves bioavailability, hinders drug metabolism, and allows for the attainment of therapeutic lopinavir concentrations. Following oral administration of lopinavir/ritonavir, maximal plasma concentrations are achieved at approximately 4.4 hours (Tmax), and the Cmax and AUCtau are 9.8 3.7 - 11.8 3.7 g/mL and 92.6 36.7 - 154.1 61.4 gh/mL, respectively. Relative to administration in the fasted state, administration with a meal increases the AUC of the tablet formulation slightly (~19%) but dramatically increases the AUC of the oral solution formulation (~130%).
Route of Elimination
Lopinavir is primarily eliminated in the feces. Following oral administration, approximately 10.4 2.3% of the administered dose is excreted in the urine and 82.6 2.5% is excreted in the feces. Unchanged parent drug accounted for 2.2% and 19.8% of the administered dose in urine and feces, respectively.
Volume of Distribution
The volume of distribution of lopinavir following oral administration is approximately 16.9 L.
Clearance
The estimated apparent clearance following oral administration is approximately 6-7 L/h.
At steady state, lopinavir is approximately 98-99% bound to plasma proteins. Lopinavir binds to both alpha-1-acid glycoprotein (AAG) and albumin; however, it has a higher affinity for AAG. At steady state, lopinavir protein binding remains constant over the range of observed concentrations after 400/100 mg KALETRA twice daily, and is similar between healthy volunteers and HIV-1 positive patients.
US Natl Inst Health; DailyMed. Current Medication Information for KALETRA (kaletra) tablet (September 2010). Available from, as of July 27, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68e860d7-4adc-418a-ba12-233ccdf85d0a
In a pharmacokinetic study in HIV-1 positive subjects (n = 19), multiple dosing with 400/100 mg KALETRA twice daily with food for 3 weeks produced a mean SD lopinavir peak plasma concentration (Cmax) of 9.8 + or - 3.7 ug/mL, occurring approximately 4 hours after administration. The mean steady-state trough concentration prior to the morning dose was 7.1 + or - 2.9 ug/mL and minimum concentration within a dosing interval was 5.5 + or - 2.7 ug/mL. Lopinavir AUC over a 12 hour dosing interval averaged 92.6 + or - 36.7 ug*h/mL. The absolute bioavailability of lopinavir co-formulated with ritonavir in humans has not been established. Under nonfasting conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of KALETRA co-formulated capsules and oral solution. When administered under fasting conditions, both the mean AUC and Cmax of lopinavir were 22% lower for the KALETRA oral solution relative to the capsule formulation.
US Natl Inst Health; DailyMed. Current Medication Information for KALETRA (kaletra) tablet (September 2010). Available from, as of July 27, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68e860d7-4adc-418a-ba12-233ccdf85d0a
Lopinavir and ritonavir are distributed into milk in rats; it is not known whether the drugs are distributed into human milk.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 636
The pharmacokinetics of once daily Kaletra have been evaluated in HIV-1 infected subjects naive to antiretroviral treatment. Kaletra 800/200 mg was administered in combination with emtricitabine 200 mg and tenofovir DF 300 mg as part of a once daily regimen. Multiple dosing of 800/200 mg Kaletra once daily for 4 weeks with food (n = 24) produced a mean + or - 3.7 SD lopinavir peak plasma concentration (Cmax) of 11.8 + or - 3.7 ug/mL, occurring approximately 6 hours after administration. The mean steady-state lopinavir trough concentration prior to the morning dose was 3.2 + or - 3.7 2.1 ug/mL and minimum concentration within a dosing interval was 1.7 + or - 3.7 1.6 ug/mL. Lopinavir AUC over a 24 hour dosing interval averaged 154.1 + or - 3.7 61.4 ug* h/mL.
US Natl Inst Health; DailyMed. Current Medication Information for KALETRA (kaletra) tablet (September 2010). Available from, as of July 27, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68e860d7-4adc-418a-ba12-233ccdf85d0a
For more Absorption, Distribution and Excretion (Complete) data for Lopinavir (11 total), please visit the HSDB record page.
Lopinavir undergoes extensive oxidative metabolism, almost exclusively via hepatic CYP3A isozymes. Co-administration with ritonavir, a potent inhibitor of CYP3A enzymes, helps to stave off lopinavir's biotransformation and increase plasma levels of active antiviral drug. Twelve metabolites have been identified _in vitro_, with the C-4 oxidation products M1, M3, and M4 being the predominant metabolites found in plasma. The structures of these primary metabolites have been identified, but precise structural information regarding the remaining minor metabolites has not been elucidated.
Lopinavir was metabolised in rat, dog and human primarily by hepatic CYP3A4 isoenzymes. Radioactivity in rat and dog faeces consisted largely of unchanged parent compound after oral administration. Although there were similarities in metabolite pattern between rat, dog and human, qualitative and quantitative differences were observed. The metabolism of lopinavir was sensitive to inhibition of ritonavir, which is in accordance with the inhibition of metabolic clearance of lopinavir by ritonavir observed in the rat.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Kaletra, Scientific Discussion p.5 (2006). Available from, as of July 27, 2013: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000368/WC500039040.pdf
In vitro experiments with human hepatic microsomes indicate that lopinavir primarily undergoes oxidative metabolism. Lopinavir is extensively metabolized by the hepatic cytochrome P450 system, almost exclusively by the CYP3A isozyme. Ritonavir is a potent CYP3A inhibitor which inhibits the metabolism of lopinavir, and therefore increases plasma levels of lopinavir. A (14)C-lopinavir study in humans showed that 89% of the plasma radioactivity after a single 400/100 mg Kaletra dose was due to parent drug. At least 13 lopinavir oxidative metabolites have been identified in man. Ritonavir has been shown to induce metabolic enzymes, resulting in the induction of its own metabolism. Pre-dose lopinavir concentrations decline with time during multiple dosing, stabilizing after approximately 10 to 16 days.
US Natl Inst Health; DailyMed. Current Medication Information for KALETRA (kaletra) tablet (September 2010). Available from, as of July 27, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68e860d7-4adc-418a-ba12-233ccdf85d0a
The elimination half-life of lopinavir is 6.9 2.2 hours.
After single dose administration, mean elimination half-life ranged between 2 to 3 hours and seemed to be increased after multiple dose administration (about 4-6 hr).
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Kaletra, Scientific Discussion p.10 (2006). Available from, as of July 27, 2013: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000368/WC500039040.pdf
The HIV lifecycle is comprised of 3 distinct stages: assembly, involving creation and packaging of essential viral components; budding, wherein the viral particle crosses the host cell plasma membrane and forms a lipid envelope; and maturation, wherein the viral particle alters its structure and becomes infectious. At the center of this lifecycle is the Gag polyprotein which, along with the products of its proteolysis, coordinate these stages and function as the major structural proteins of the virus. The HIV-1 protease enzyme, a dimeric aspartic protease, is the enzyme responsible for cleaving the Gag polyprotein and thus plays a critical role in many aspects of the HIV viral lifecycle. Lopinavir is an inhibitor of the HIV-1 protease enzyme. Its design is based on the "peptidomimetic" principle, wherein the molecule contains a hydroxyethylene scaffold which mimics the normal peptide linkage (cleaved by HIV protease) but which itself cannot be cleaved. By preventing HIV-1 protease activity, and thus the proteolysis of the Gag polyprotein, lopinavir results in the production of immature, non-infectious viral particles.
/The researchers/ have previously shown that the HIV protease inhibitor lopinavir has selective toxicity against human papillomavirus (HPV)-positive cervical carcinoma cells via an unknown mechanism. SiHa cervical carcinoma cells were stably transfected with the proteasome sensor vector pZsProSensor-1 to confirm lopinavir inhibits the proteasome in these cells. The Panorama Xpress profiler 725 antibody array was then used to analyse specific changes in protein expression in lopinavir-treated versus control untreated SiHa cells followed by PCR and western blotting. Colorimetric growth assays of lopinavir-treated E6/E7 immortalised versus control human keratinocytes were performed. Targeted small interfering RNA gene silencing followed by growth assay comparison of lopinavir-treated/untreated SiHa cells was also used. Lopinavir induced an increase in the fluorescence of pZsProSensor-1 transfected SiHa cells, indicative of proteasomal inhibition. Ribonuclease L (RNASEL) protein was shown to be up-regulated in lopinavir-treated SiHa cells, which was confirmed by PCR and western blot. Targeted silencing of RNASEL reduced the sensitivity of SiHa cells to lopinavir. Selective toxicity against E6/E7 immortalised keratinocytes versus control cells was also seen with lopinavir and was associated with up-regulated RNASEL expression. These data are consistent with the toxicity of lopinavir against HPV-positive cervical carcinoma cells being related to its ability to block viral proteasome activation and induce an up-regulation of the antiviral protein RNASEL. This is supported by the drug's selective toxicity and up-regulation of RNASEL in E6/E7 immortalised keratinocytes combined with the increased resistance to lopinavir observed in SiHa cells following silencing of RNASEL gene expression.
PMID:21685539 Batman G et al; Antivir Ther 16 (4): 515-25 (2011)
Lopinavir inhibits replication of HIV type 1 (HIV-1) by interfering with HIV protease. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes to form structural proteins of the virion core and essential viral enzymes. By interfering with the formation of these essential proteins and enzymes, lopinavir blocks maturation of the virus and causes formation of nonfunctional, immature, noninfectious virions. Lopinavir also has some in vitro activity against HIV type 2 (HIV-2).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 639

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
28
PharmaCompass offers a list of Lopinavir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lopinavir manufacturer or Lopinavir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lopinavir manufacturer or Lopinavir supplier.
PharmaCompass also assists you with knowing the Lopinavir API Price utilized in the formulation of products. Lopinavir API Price is not always fixed or binding as the Lopinavir Price is obtained through a variety of data sources. The Lopinavir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lopinavir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lopinavir, including repackagers and relabelers. The FDA regulates Lopinavir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lopinavir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lopinavir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lopinavir supplier is an individual or a company that provides Lopinavir active pharmaceutical ingredient (API) or Lopinavir finished formulations upon request. The Lopinavir suppliers may include Lopinavir API manufacturers, exporters, distributors and traders.
click here to find a list of Lopinavir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lopinavir DMF (Drug Master File) is a document detailing the whole manufacturing process of Lopinavir active pharmaceutical ingredient (API) in detail. Different forms of Lopinavir DMFs exist exist since differing nations have different regulations, such as Lopinavir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lopinavir DMF submitted to regulatory agencies in the US is known as a USDMF. Lopinavir USDMF includes data on Lopinavir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lopinavir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lopinavir suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lopinavir Drug Master File in Korea (Lopinavir KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lopinavir. The MFDS reviews the Lopinavir KDMF as part of the drug registration process and uses the information provided in the Lopinavir KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lopinavir KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lopinavir API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lopinavir suppliers with KDMF on PharmaCompass.
A Lopinavir CEP of the European Pharmacopoeia monograph is often referred to as a Lopinavir Certificate of Suitability (COS). The purpose of a Lopinavir CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lopinavir EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lopinavir to their clients by showing that a Lopinavir CEP has been issued for it. The manufacturer submits a Lopinavir CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lopinavir CEP holder for the record. Additionally, the data presented in the Lopinavir CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lopinavir DMF.
A Lopinavir CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lopinavir CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lopinavir suppliers with CEP (COS) on PharmaCompass.
A Lopinavir written confirmation (Lopinavir WC) is an official document issued by a regulatory agency to a Lopinavir manufacturer, verifying that the manufacturing facility of a Lopinavir active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lopinavir APIs or Lopinavir finished pharmaceutical products to another nation, regulatory agencies frequently require a Lopinavir WC (written confirmation) as part of the regulatory process.
click here to find a list of Lopinavir suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lopinavir as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lopinavir API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lopinavir as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lopinavir and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lopinavir NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lopinavir suppliers with NDC on PharmaCompass.
Lopinavir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lopinavir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lopinavir GMP manufacturer or Lopinavir GMP API supplier for your needs.
A Lopinavir CoA (Certificate of Analysis) is a formal document that attests to Lopinavir's compliance with Lopinavir specifications and serves as a tool for batch-level quality control.
Lopinavir CoA mostly includes findings from lab analyses of a specific batch. For each Lopinavir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lopinavir may be tested according to a variety of international standards, such as European Pharmacopoeia (Lopinavir EP), Lopinavir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lopinavir USP).
