
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
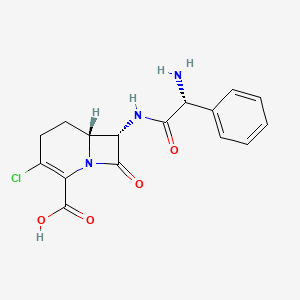

1. Carbac
2. Kt 3777
3. Kt-3777
4. Lorabid
5. Loracarbef Monohydrate
6. Lorafem
7. Lorax
8. Ly 163892
9. Ly-163892
10. Ly163892
1. 76470-66-1
2. Loracarbefum
3. Loracarbef Anhydrous
4. Loracarbefum [inn-latin]
5. Anhydrous Loracarbef
6. Loracarbef, Anhydrous
7. W72i5zt78z
8. (6r,7s)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
9. Chebi:47544
10. Loracarbef (inn)
11. Ly-163892
12. 1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid, 7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-, (6r,7s)-
13. Loracarbef [inn]
14. (6r,7s)-7-{[(2r)-2-amino-2-phenylacetyl]amino}-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
15. Kt 3777
16. Unii-w72i5zt78z
17. Ly163892
18. Lorbef
19. Loracarbef [usan:inn:ban]
20. Ly 163892
21. Lorbef (tn)
22. Loracabef
23. Loracarbef [mi]
24. Chembl1013
25. Loracarbef [who-dd]
26. Schembl34153
27. Bidd:gt0753
28. Dtxsid7023223
29. Hms3713l04
30. Hy-b1682
31. Zinc1530993
32. Akos015895936
33. Ccg-220611
34. Db00447
35. 7beta-[(2r)-2-amino-2-phenylacetyl]nitrilo-3-chloro-3,4-didehydrocepham-4-carboxylic Acid
36. Ncgc00510749-15
37. 1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-chloro-8-oxo-, (6r-(6alpha,7beta(r*)))-
38. Cs-0013651
39. D08143
40. Q979521
41. Brd-k28935038-001-01-4
42. (6r,7s)-7-[(2r)-2-amino-2-phenylacetamido]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
43. 1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-aminophenylacetyl)amino)-3-chloro-8-oxo-, (6r,7s)-
| Molecular Weight | 349.77 g/mol |
|---|---|
| Molecular Formula | C16H16ClN3O4 |
| XLogP3 | -1.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 349.0829337 g/mol |
| Monoisotopic Mass | 349.0829337 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to treat upper respiratory tract bacterial infections, chronic bronchitis, pneumonia, sinusitis, pharyntitis and tonsillitis, skin absceses, urinary tract infections and pyelonephritis caused by E. coli, S. pyogenes, S. aureus, S. saprphyticus, S. penumoniae, H. influenzae and M. catarrhalis.
Loracarbef is considered a second generation cephalosporin antibiotic. The advantages of cephalosporin antibiotics include a broad range of activity, a safe record in children with almost no dose-related toxicity, and the lack of need to monitor levels. Adverse reactions are rare and consist primarily of hypersensitivity reactions with urticaria, nonspecific rash, and pruritus. Loracarbef can be used to treat a large number of bacterial infections caused by gram-negative and gram-positive bacteria, including upper respiratory tract bacterial infections, chronic bronchitis, pneumonia, sinusitis, pharyntitis and tonsillitis, skin absceses, urinary tract infections and pyelonephritis caused by E. coli, S. pyogenes, S. aureus, S. saprphyticus, S. penumoniae, H. influenzae and M. catarrhalis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC08 - Loracarbef
Absorption
Well absorbed with approximately 90% absorbed from the gastrointestinal tract after oral ingestion.
There is no evidence of metabolism in humans.
1 hour. In subjects with moderate impairment of renal function the plasma half-life was prolonged to approximately 5.6 hours.
Loracarbef is an oral, synthetic beta-lactam antibiotic of the carbacephem class. Chemically, carbacephems differ from cephalosporin-class antibiotics in the dihydrothiazine ring where a methylene group has been substituted for a sulfur atom. Loracarbef has a spectrum of activity similar to that of the second generation cephalosporins. It is structurally identical to cefaclor except for a sulfur atom that has been replaced by a methylene group. This change gives greater chemical stability in solution and allows storage at room temperature. Loracarbef, like all b-lactams and cephalosporins, inhibits penicillin binding proteins, enzymes that create the cross-linkage of the peptidoglycan polymer. This binding leads to interference with the formation and remodeling of the cell wall structure.
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?