
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
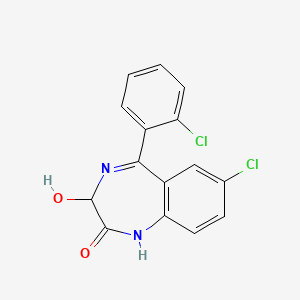

1. Apo Lorazepam
2. Apo-lorazepam
3. Ativan
4. Donix
5. Duralozam
6. Durazolam
7. Idalprem
8. Laubeel
9. Lorazep Von Ct
10. Lorazepam Medical
11. Lorazepam Neuraxpharm
12. Lorazepam Ratiopharm
13. Lorazepam-neuraxpharm
14. Lorazepam-ratiopharm
15. Medical, Lorazepam
16. Novo Lorazem
17. Novo-lorazem
18. Nu Loraz
19. Nu-loraz
20. Orfidal Wyeth
21. Sedicepan
22. Sinestron
23. Somagerol
24. Tmesta
25. Temesta
26. Tolid
27. Von Ct, Lorazep
28. Wy 4036
29. Wy-4036
30. Wy4036
31. Wyeth, Orfidal
1. Ativan
2. 846-49-1
3. O-chloroxazepam
4. O-chlorooxazepam
5. Temesta
6. Loraz
7. Tavor
8. Delormetazepam
9. Almazine
10. Anxiedin
11. Bonatranquan
12. Duralozam
13. Emotival
14. Lorabenz
15. Sedatival
16. Laubeel
17. Orfidal
18. Punktyl
19. Lorax
20. Quait
21. Wypax
22. Apo-lorazepam
23. Lorazepam Intensol
24. Norlormetazepam
25. Anzepam
26. Aplacasse
27. Azurogen
28. Efasedan
29. Equitam
30. Kalmalin
31. Larpose
32. Lorapam
33. Lorazene
34. Lorazep
35. Lorazin
36. Lorenin
37. Loridem
38. Lorsedal
39. Lozepam
40. Novhepar
41. Renaquil
42. Rocosgen
43. Sedizepan
44. Sidenar
45. Silence
46. Tranqipam
47. Anxira
48. Aripax
49. Bonton
50. Loram
51. Lorat
52. Merlit
53. Stapam
54. Trapax
55. Upan
56. Nervistop L
57. Wy-4036
58. Demethyllormetazepam
59. Lorazepamum
60. Wy 4036
61. (+/-)-lorazepam
62. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one
63. Lorazepam Preservative Free
64. Lorazepam Civ
65. 2h-1,4-benzodiazepin-2-one, 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-
66. Nsc 289758
67. Idalprem
68. 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2h-1,4-benzodiazepin-2-one
69. Aplacassee
70. Sinestron
71. Donix
72. Tolid
73. Chembl580
74. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1h-1,4-benzodiazepin-2(3h)-one
75. Lorazepam Medical
76. Nu Loraz
77. 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2h-1,4-benzodiazepin-2-one
78. O26fzp769l
79. Nervistopl
80. Novolorazem
81. Emotion
82. Lomesta
83. Lorafen
84. Lorazon
85. Lorivan
86. Somagerol
87. Vigiten
88. Lorans
89. Lorzem
90. Lopam
91. Lorazepam Fabra
92. Lorazepam-efeka
93. Lorazepan Chobet
94. Lorazepan Richet
95. Max Pax
96. Nsc289758
97. Lorazepam Genericon
98. Lorazepam Lannacher
99. Nsc-289758
100. Ncgc00159439-02
101. Lorsilan
102. Securit
103. Sedazin
104. Pro Dorm
105. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-2h-1,4-benzodiazepin-2-one
106. 2h-1,4-benzodiazepin-2-one, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-
107. Lorazepamum [inn-latin]
108. Ativan (tn)
109. Einecs 212-687-6
110. Brn 0759084
111. Unii-o26fzp769l
112. Dea No. 2885
113. Lorazepam [usan:usp:inn:ban:jan]
114. Loreev Xr
115. Lorazepam [inn]
116. Lorazepam [jan]
117. Lorazepam [mi]
118. Lorazepam [usan]
119. (.+/-.)-lorazepam
120. Lorazepam [vandf]
121. Dsstox_cid_3225
122. Lorazepam [mart.]
123. Lorazepam [who-dd]
124. (+-)-7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2h-1,4-benzodiazepin-2-one
125. Dsstox_rid_76932
126. Dsstox_gsid_23225
127. Schembl26961
128. 5-25-02-00248 (beilstein Handbook Reference)
129. Mls001424236
130. Divk1c_000965
131. Lorazepam (jp17/usp/inn)
132. Chebi:6539
133. Gtpl5884
134. Lorazepam [ep Impurity]
135. Lorazepam [orange Book]
136. Lorazepam Civ [usp-rs]
137. Dtxsid7023225
138. Lorazepam [ep Monograph]
139. Hms503a11
140. Kbio1_000965
141. Lorazepam [usp Monograph]
142. Ninds_000965
143. Hms2052h07
144. Hms3394h07
145. Bcp13713
146. Tox21_111669
147. Bdbm50292627
148. Lorazepam 0.1 Mg/ml In Acetonitrile
149. Lorazepam 1.0 Mg/ml In Acetonitrile
150. Akos015904303
151. Ccg-101170
152. Db00186
153. Nc00420
154. 2h-1,4-benzodiazepin-2-one, 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-, (+-)-
155. Idi1_000965
156. Cas-846-49-1
157. Smr000058410
158. Wln: T67 Gmv Jn Ihj Cg Iq Kr Bg
159. L-230
160. D00365
161. 846l491
162. A840886
163. Q408265
164. 7-chloro-5-(o-chlorophenyl)-1,4-benzodiazepin-2-one
165. Brd-a11990600-001-01-8
166. Lorazepam, British Pharmacopoeia (bp) Reference Standard
167. Lorazepam, European Pharmacopoeia (ep) Reference Standard
168. 2h-1, 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-
169. 2h-1, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-
170. Lorazepam, United States Pharmacopeia (usp) Reference Standard
171. O-chloroxazepam; O-chlorooxazepam; Almazine; Wy 4036; Wy-4036; Wy4036
172. 7-chloranyl-5-(2-chlorophenyl)-3-oxidanyl-1,3-dihydro-1,4-benzodiazepin-2-one
173. 7-chloro-3-hydroxy-5-(2'-chloro-phenyl)-1,3-dihydro-2h-1,4-benzodiazepine-2-one
174. 7-chloro-3-hydroxy-5-(2'-chlorophenyl)-1,3-dihydro-2h-1,4-benzodiazepine-2-one
175. 7-chloro-5-(2-chloro-phenyl)-3-hydroxy-1,3-dihydro-benzo[e][1,4]diazepin-2-one
176. 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2h-1,4-benzodiazepine-2-one
177. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one.
178. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-2h-1,4-benzodiazepin-2-one #
179. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1h-benzo[e][1,4]diazepin-2(3h)-one
180. 7-chloro-5-(2-chlorophenyl)-3-hydroxy-2,3-dihydro-1h-1,4-benzodiazepin-2-one
181. Lorazepam For System Suitability, European Pharmacopoeia (ep) Reference Standard
182. Lorazepam Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
183. Lorazepam7-chloro-5-(2-chloro-phenyl)-3-hydroxy-1,3-dihydro-benzo[e][1,4]diazepin-2-one
184. (+/-)-7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2h-1,4-benzodiazepin-2-one
185. (rs)-9-chloro-6-(2-chlorophenyl)-4-hydroxy-2,5-diazabicyclo[5.4.0]undeca-5,8,10,12-tetraen-3-one
186. 2h-1,4-benzodiazepin-2-one, 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-, (+/-)-
187. 7-chloro-5-(2-chloro-phenyl)-3-hydroxy-1,3-dihydro-benzo[e][1,4]diazepin-2-one (lorazepam)
| Molecular Weight | 321.2 g/mol |
|---|---|
| Molecular Formula | C15H10Cl2N2O2 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 320.0119330 g/mol |
| Monoisotopic Mass | 320.0119330 g/mol |
| Topological Polar Surface Area | 61.7 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 443 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Ativan |
| PubMed Health | Lorazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Lorazepam, a benzodiazepine with antianxiety, sedative, and anticonvulsant effects, is intended for the intramuscular or intravenous routes of administration. It has the chemical formula: 7-chloro-5(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1, 4-benzo... |
| Active Ingredient | Lorazepam |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 2mg/ml; 4mg/ml; 0.5mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Valeant Intl; Hikma Maple |
| 2 of 8 | |
|---|---|
| Drug Name | Lorazepam |
| PubMed Health | Lorazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one:It is a nearly white powder almost insoluble in water. Each lorazepam tablet, to be taken orally, contains 0.5 mg,... |
| Active Ingredient | Lorazepam |
| Dosage Form | Tablet; Concentrate; Injectable |
| Route | Injection; Oral |
| Strength | 2mg/ml; 0.5mg; 1mg; 4mg/ml; 2mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Amneal Pharms; Ranbaxy; Excellium; Hi-tech Pharma; Hospira; Pharm Assoc; Lupin; Sandoz; Watson Labs; Ivax Sub Teva Pharms; Intl Medication Sys; Paddock; Mylan; Akorn |
| 3 of 8 | |
|---|---|
| Drug Name | Lorazepam intensol |
| PubMed Health | Lorazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Active Ingredient | Lorazepam |
| Dosage Form | Concentrate |
| Route | Oral |
| Strength | 2mg/ml |
| Market Status | Prescription |
| Company | Roxane |
| 4 of 8 | |
|---|---|
| Drug Name | Lorazepam preservative free |
| Active Ingredient | Lorazepam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml; 4mg/ml |
| Market Status | Prescription |
| Company | Bedford Labs |
| 5 of 8 | |
|---|---|
| Drug Name | Ativan |
| PubMed Health | Lorazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Lorazepam, a benzodiazepine with antianxiety, sedative, and anticonvulsant effects, is intended for the intramuscular or intravenous routes of administration. It has the chemical formula: 7-chloro-5(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1, 4-benzo... |
| Active Ingredient | Lorazepam |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 2mg/ml; 4mg/ml; 0.5mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Valeant Intl; Hikma Maple |
| 6 of 8 | |
|---|---|
| Drug Name | Lorazepam |
| PubMed Health | Lorazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one:It is a nearly white powder almost insoluble in water. Each lorazepam tablet, to be taken orally, contains 0.5 mg,... |
| Active Ingredient | Lorazepam |
| Dosage Form | Tablet; Concentrate; Injectable |
| Route | Injection; Oral |
| Strength | 2mg/ml; 0.5mg; 1mg; 4mg/ml; 2mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Amneal Pharms; Ranbaxy; Excellium; Hi-tech Pharma; Hospira; Pharm Assoc; Lupin; Sandoz; Watson Labs; Ivax Sub Teva Pharms; Intl Medication Sys; Paddock; Mylan; Akorn |
| 7 of 8 | |
|---|---|
| Drug Name | Lorazepam intensol |
| PubMed Health | Lorazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Active Ingredient | Lorazepam |
| Dosage Form | Concentrate |
| Route | Oral |
| Strength | 2mg/ml |
| Market Status | Prescription |
| Company | Roxane |
| 8 of 8 | |
|---|---|
| Drug Name | Lorazepam preservative free |
| Active Ingredient | Lorazepam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml; 4mg/ml |
| Market Status | Prescription |
| Company | Bedford Labs |
Lorazepam is FDA-approved for the short-term relief of anxiety symptoms related to anxiety disorders and anxiety associated with depressive symptoms such as anxiety-associated insomnia. It is as well used as an anesthesia premedication in adults to relieve anxiety or to produce sedation/amnesia and for the treatment of status epilepticus. Some off-label indications of lorazepam include rapid tranquilization of an agitated patient, alcohol withdrawal delirium, alcohol withdrawal syndrome, muscle spasms, insomnia, panic disorder, delirium, chemotherapy-associated anticipatory nausea and vomiting, and psychogenic catatonia.
FDA Label
The effect of lorazepam in GABA-A receptors produces an increase in the frequency of opening of the chloride ion channel. However, for its effect to generate, the neurotransmitter is required. The anticonvulsant properties of lorazepam are thought to be related to the binding to voltage-dependent sodium channels in which the sustained repetitive firing gets limited by the slow recovery of sodium channels due to the benzodiazepine effect. The effect of lorazepam seems to be very compartmental which was observed with a different generation of sleepiness and a dizziness effect.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
N05BA06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA06 - Lorazepam
Absorption
Readily absorbed with an absolute bioavailability of 90% when given orally. When intramuscularly administered a dose of 4 mg, lorazepam is completely and rapidly absorbed and achieves a maximal serum concentration of 48 ng/ml in 15-30 minutes. When administered orally, the time to attained maximum concentration is observed to be of 2 hours.
Route of Elimination
When a single 2 mg oral dose is given to healthy subjects, 88% of the administered dose is recovered in urine and 7% was recovered in feces. From the excreted dose in urine, the major form is the glucuronide version that represents 74% while only 0.3% of the dose is recovered as unchanged lorazepam.
Volume of Distribution
The reported volume of distribution of lorazepam is 1.3 L/kg. It is important to mention that due to the lipophilicity of lorazepam, it does not redistribute as fast in the brain.
Clearance
_In vivo_ studies with lorazepam have shown a clearance rate of 5.8 ml.min/kg.
Lorazepam is hepatically metabolized by CYP450 isoenzymes and extensively conjugated to the 3-0-phenolic glucuronide. This is an inactive metabolite and is eliminated mainly by the kidneys.
Lorazepam has known human metabolites that include Lorazepam glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
When administered parentally, the registered half-life of lorazepam is of 14 hours.
Lorazepam allosterically binds on the benzodiazepine receptors in the post-synaptic GABA-A ligand-gated chloride channel in different sites of the central nervous system (CNS). This binding will result in an increase on the GABA inhibitory effects which is translated as an increase in the flow of chloride ions into the cell causing hyperpolarization and stabilization of the cellular plasma membrane. According to the binding site of lorazepam, we can observe different activities as the binding in the amygdala is known to help mainly in anxiety disorders while the binding in the cerebral cortex helps in seizure disorders.
 Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23247
Submission : 2009-11-05
Status : Active
Type : II

Date of Issue : 2022-06-08
Valid Till : 2025-07-14
Written Confirmation Number : WC-0093
Address of the Firm :

NDC Package Code : 57218-950
Start Marketing Date : 2009-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5041
Submission : 1983-07-09
Status : Active
Type : II

Certificate Number : R1-CEP 2008-142 - Rev 04
Issue Date : 2021-03-30
Type : Chemical
Substance Number : 1121
Status : Valid

NDC Package Code : 52696-0002
Start Marketing Date : 1983-07-09
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.

NDC Package Code : 58159-103
Start Marketing Date : 2024-08-20
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (35kg/35kg)
Marketing Category : BULK INGREDIENT
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19268
Submission : 2006-03-21
Status : Inactive
Type : II

GDUFA
DMF Review : Reviewed
Rev. Date : 2021-01-29
Pay. Date : 2020-12-08
DMF Number : 35299
Submission : 2020-12-11
Status : Active
Type : II

NDC Package Code : 72166-001
Start Marketing Date : 2018-04-09
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-01-17
Pay. Date : 2023-01-13
DMF Number : 19006
Submission : 2005-11-28
Status : Active
Type : II

Certificate Number : CEP 2010-217 - Rev 03
Issue Date : 2024-01-25
Type : Chemical
Substance Number : 1121
Status : Valid
Date of Issue : 2020-03-31
Valid Till : 2022-07-28
Written Confirmation Number : WC-0445A3
Address of the Firm :

NDC Package Code : 71554-009
Start Marketing Date : 2019-04-27
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15156
Submission : 2000-11-21
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23247
Submission : 2009-11-05
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5041
Submission : 1983-07-09
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19268
Submission : 2006-03-21
Status : Inactive
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-09-25
Pay. Date : 2020-09-21
DMF Number : 4517
Submission : 1982-05-04
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-09-29
Pay. Date : 2013-09-23
DMF Number : 22460
Submission : 2009-01-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17765
Submission : 2004-10-20
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-06-27
Pay. Date : 2014-06-20
DMF Number : 5027
Submission : 1983-05-17
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-01-17
Pay. Date : 2023-01-13
DMF Number : 19006
Submission : 2005-11-28
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5747
Submission : 1985-03-13
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15156
Submission : 2000-11-21
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Number : R1-CEP 2008-142 - Rev 04
Status : Valid
Issue Date : 2021-03-30
Type : Chemical
Substance Number : 1121
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-049 - Rev 02
Status : Valid
Issue Date : 2022-04-19
Type : Chemical
Substance Number : 1121

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2009-056 - Rev 03
Status : Valid
Issue Date : 2023-10-19
Type : Chemical
Substance Number : 1121

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2016-243 - Rev 00
Status : Valid
Issue Date : 2022-12-16
Type : Chemical
Substance Number : 1121

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2012-113 - Rev 01
Status : Valid
Issue Date : 2025-02-10
Type : Chemical
Substance Number : 1121

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2010-217 - Rev 03
Status : Valid
Issue Date : 2024-01-25
Type : Chemical
Substance Number : 1121

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
42
PharmaCompass offers a list of Lorazepam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lorazepam manufacturer or Lorazepam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lorazepam manufacturer or Lorazepam supplier.
PharmaCompass also assists you with knowing the Lorazepam API Price utilized in the formulation of products. Lorazepam API Price is not always fixed or binding as the Lorazepam Price is obtained through a variety of data sources. The Lorazepam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lorazepam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lorazepam, including repackagers and relabelers. The FDA regulates Lorazepam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lorazepam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lorazepam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lorazepam supplier is an individual or a company that provides Lorazepam active pharmaceutical ingredient (API) or Lorazepam finished formulations upon request. The Lorazepam suppliers may include Lorazepam API manufacturers, exporters, distributors and traders.
click here to find a list of Lorazepam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lorazepam DMF (Drug Master File) is a document detailing the whole manufacturing process of Lorazepam active pharmaceutical ingredient (API) in detail. Different forms of Lorazepam DMFs exist exist since differing nations have different regulations, such as Lorazepam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lorazepam DMF submitted to regulatory agencies in the US is known as a USDMF. Lorazepam USDMF includes data on Lorazepam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lorazepam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lorazepam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lorazepam Drug Master File in Japan (Lorazepam JDMF) empowers Lorazepam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lorazepam JDMF during the approval evaluation for pharmaceutical products. At the time of Lorazepam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lorazepam suppliers with JDMF on PharmaCompass.
A Lorazepam CEP of the European Pharmacopoeia monograph is often referred to as a Lorazepam Certificate of Suitability (COS). The purpose of a Lorazepam CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lorazepam EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lorazepam to their clients by showing that a Lorazepam CEP has been issued for it. The manufacturer submits a Lorazepam CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lorazepam CEP holder for the record. Additionally, the data presented in the Lorazepam CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lorazepam DMF.
A Lorazepam CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lorazepam CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lorazepam suppliers with CEP (COS) on PharmaCompass.
A Lorazepam written confirmation (Lorazepam WC) is an official document issued by a regulatory agency to a Lorazepam manufacturer, verifying that the manufacturing facility of a Lorazepam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lorazepam APIs or Lorazepam finished pharmaceutical products to another nation, regulatory agencies frequently require a Lorazepam WC (written confirmation) as part of the regulatory process.
click here to find a list of Lorazepam suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lorazepam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lorazepam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lorazepam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lorazepam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lorazepam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lorazepam suppliers with NDC on PharmaCompass.
Lorazepam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lorazepam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lorazepam GMP manufacturer or Lorazepam GMP API supplier for your needs.
A Lorazepam CoA (Certificate of Analysis) is a formal document that attests to Lorazepam's compliance with Lorazepam specifications and serves as a tool for batch-level quality control.
Lorazepam CoA mostly includes findings from lab analyses of a specific batch. For each Lorazepam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lorazepam may be tested according to a variety of international standards, such as European Pharmacopoeia (Lorazepam EP), Lorazepam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lorazepam USP).
