
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
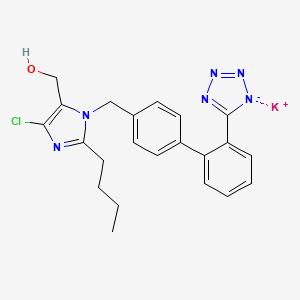

1. 2-butyl-4-chloro-1-((2'-(1h-etrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl)-1h-imidazole-5-methanol
2. Cozaar
3. Dup 753
4. Dup-753
5. Dup753
6. Losartan
7. Losartan Monopotassium Salt
8. Mk 954
9. Mk-954
10. Mk954
11. Monopotassium Salt, Losartan
12. Potassium, Losartan
13. Salt, Losartan Monopotassium
1. 124750-99-8
2. Cozaar
3. Losartan Potassium Salt
4. Lorzaar
5. Losacar
6. Losaprex
7. Hyzaar
8. Dup 753
9. Nu-lotan
10. Mk 954
11. Lortaan
12. Losata
13. Tancin
14. Mk-0954
15. Losartanpotassium
16. Losartan Potassium (dup 753)
17. Mk0954
18. L-158086
19. Losartan Monopotassium Salt
20. 3st302b24a
21. Aradois
22. Zaart
23. Dup-753
24. Presartan-50
25. E-3340
26. 2-butyl-4-chloro-1-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)imidazole-5-methanol, Monopotassium Salt
27. 124750-99-8 (ka+)
28. Potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol
29. Lifezar
30. Lorzaan
31. Losacor
32. Tenopres
33. Lotim
34. Niten
35. Ocsaar
36. Du Pont-753
37. Neo Lotan
38. 1h-imidazole-5-methanol, 2-butyl-4-chloro-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, Monopotassium Salt
39. 2-butyl-4-chloro-1-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-imidazole-5-methanol, Monopotassium Salt
40. Mfcd09850721
41. Mk-954
42. Du Pont 753
43. Losartan Potassium [usan]
44. Covance
45. Unii-3st302b24a
46. Losartan Potassium [usan:usp]
47. Potassium 5-(4'-((2-butyl-4-chloro-5-(hydroxymethyl)-1h-imidazol-1-yl)methyl)biphenyl-2-yl)tetrazol-1-ide
48. Cozaar (tn)
49. Dup-753 Potassium
50. Mk 0954
51. Losartan Potassium,(s)
52. Schembl42079
53. Mls001401407
54. Ex-89
55. Dtxsid3044209
56. Losartan Potassium [jan]
57. Losartan Potassium (jp17/usp)
58. Losartan Potassium [hsdb]
59. Hms2051m12
60. Hms2090o22
61. Hms2235f20
62. Hms3369f08
63. Hms3393m12
64. Losartan Potassium [vandf]
65. Losartan Potassium [mart.]
66. Act02618
67. Bcp05332
68. Bcp29397
69. Losartan Potassium [usp-rs]
70. Losartan Potassium [who-dd]
71. Akos015955543
72. Akos025310168
73. Ac-1072
74. Ccg-100869
75. Losartan Potassium, Analytical Standard
76. Nc00119
77. 2-butyl-4-chloro-1-(2'-(tetrazol-5-yl)biphenyl-4-ylmethyl)-1h-imidazole-5-methanol Potassium
78. Losartan Monopotassium Salt [mi]
79. Losartan Potassium [orange Book]
80. Losartan Potassium [ep Monograph]
81. 2-butyl-4-chloro-1-[[2'-(1h-tetrazol-5-yl)-1,1'-biphenyl-4-yl]methyl]imidazole-5-methanol Potassium Salt
82. Bl164642
83. Epo
84. Hyzaar Component Losartan Potassium
85. Losartan Potassium [usp Monograph]
86. Smr000469593
87. Ft-0625705
88. L-185
89. L0232
90. Losartan Potassium Component Of Hyzaar
91. D00357
92. Ab01275507-01
93. 750l998
94. A805291
95. Sr-05000001514
96. Sr-05000001514-1
97. Q27257991
98. Losartan Potassium, European Pharmacopoeia (ep) Reference Standard
99. Losartan Potassium Is Known As A Potent, Synthetic At1 Receptor Antagonist.
100. Losartan Potassium, United States Pharmacopeia (usp) Reference Standard
101. Losartan Potassium, Pharmaceutical Secondary Standard; Certified Reference Material
102. [2-butyl-5-chloro-3-[[4-[2-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol; Potassium;losartan Potassium
103. 1h-imidazole-5-methanol, 2-butyl-4-chloro-1-((2-(1h-tetrazol-5-yl)(1,1-biphenyl)-4-yl)methyl)-, Monopotassium Salt
104. Potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol.
| Molecular Weight | 461.0 g/mol |
|---|---|
| Molecular Formula | C22H22ClKN6O |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 460.1180685 g/mol |
| Monoisotopic Mass | 460.1180685 g/mol |
| Topological Polar Surface Area | 77.7 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 526 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Cozaar |
| PubMed Health | Losartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | COZAAR1(losartan potassium) is an angiotensin II receptor (type AT1) antagonist. Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt.Its... |
| Active Ingredient | Losartan potassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 2 of 4 | |
|---|---|
| Drug Name | Losartan potassium |
| Drug Label | Losartan potassium tablets USP are an angiotensin II receptor (type AT1) antagonist. Losartan potassium, USP a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium... |
| Active Ingredient | Losartan potassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Upsher Smith; Teva; Apotex; Alembic Pharms; Aurobindo Pharma; Torrent Pharms; Lupin; Sandoz; Prinston; Roxane; Watson Labs; Macleods Pharms; Ipca Labs; Vivimed Labs; Micro Labs Ltd India; Cadista Pharms; Zydus Pharms Usa; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Cozaar |
| PubMed Health | Losartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | COZAAR1(losartan potassium) is an angiotensin II receptor (type AT1) antagonist. Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt.Its... |
| Active Ingredient | Losartan potassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 4 of 4 | |
|---|---|
| Drug Name | Losartan potassium |
| Drug Label | Losartan potassium tablets USP are an angiotensin II receptor (type AT1) antagonist. Losartan potassium, USP a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium... |
| Active Ingredient | Losartan potassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Upsher Smith; Teva; Apotex; Alembic Pharms; Aurobindo Pharma; Torrent Pharms; Lupin; Sandoz; Prinston; Roxane; Watson Labs; Macleods Pharms; Ipca Labs; Vivimed Labs; Micro Labs Ltd India; Cadista Pharms; Zydus Pharms Usa; Mylan |
Indicated in adult and paediatric patients for the: - treatment of anemia due to Chronic Kidney Disease (CKD) in patients on dialysis and not on dialysis. - treatment of anemia due to zidovudine in patients with HIV-infection. - treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy. - reduction of allogeneic RBC transfusions in patients undergoing elective, noncardiac, nonvascular surgery.
FDA Label
Proteinuria, Treatment of heart failure, Treatment of hypertension
Erythropoietin and epoetin alfa are involved in the regulation of erythrocyte differentiation and the maintenance of a physiological level of circulating erythrocyte mass. It is reported to increase the reticulocyte count within 10 days of initiation, followed by increases in the RBC count, hemoglobin, and hematocrit, usually within 2 to 6 weeks. Depending on the dose administered, the rate of hemoglobin increase may vary. In patients receiving hemodialysis, a greater biologic response is not observed at doses exceeding 300 Units/kg 3 times weekly. Epoetin alfa serves to restore erythropoietin deficiency in pathological and other clinical conditions where normal production of erythropoietin is impaired or compromised. In anemic patients with chronic renal failure (CRF), administration with epoetin alfa stimulated erythropoiesis by increasing the reticulocyte count within 10 days, followed by increases in the red cell count, hemoglobin, and hematocrit, usually within 2 to 6 weeks. Epoetin alfa was shown to be effective in increasing hematocrit in zidovudine-treated HIV-infected patients and anemic cancer patients undergoing chemotherapy.
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
B - Blood and blood forming organs
B03 - Antianemic preparations
B03X - Other antianemic preparations
B03XA - Other antianemic preparations
B03XA01 - Erythropoietin
Absorption
The time to reach peak concentration is slower via the subcutaneous route than the intravenous route which ranges from 20 to 25 hours, and the peak is always well below the peak achieved using the intravenous route (510% of those seen with IV administration). The bioavailability of subcutaneous injectable erythropoietin is much lower than that of the intravenously administered product and is approximately 20-40%. **Adult and paediatric patients with CRF:** Following subcutaneous administration, the peak plasma levels are achieved within 5 to 24 hours. **Cancer patients receiving cyclic chemotherapy:** The average time to reach peak plasma concentration was approximately 13.3 12.4 hours after 150 Units/kg three times per week (TIW) subcutaneous (SC) dosing. The Cmax is expected be 3- to 7- fold higher and the Tmax is expected to be 2- to 3-fold longer in patients receiving a 40,000 Units SC weekly dosing regimen.
Route of Elimination
Erythropoietin and epoetin alfa are cleared via uptake and degradation via the EPO-R-expressing cells, and may also involve other cellular pathways in the interstitium, probably via cells in the reticuloendothelial scavenging pathway or lymphatic system. Only a small amount of unchanged epoetin alfa is found in the urine.
Volume of Distribution
In healthy volunteers, the volume of distribution of intravenous epoetin alfa was generally similar to the plasma volume (range of 4063.80 mL/kg), indicating limited extravascular distribution.
Clearance
**Healthy volunteers: *
In male volunteers receiving intravenous epoetin alfa, the total body clearance was approximately 8.12 1.00 mL/h/kg. **Cancer patients receiving cyclic chemotherapy:*
The average clearance was approximately 20.2 15.9 mL/h/kg after 150 Units/kg three times per week (TIW) subcutaneous (SC) dosing. The patients receiving a 40,000 Units SC weekly dosing regimen display a lower clearance (9.2 4.7 mL/h/kg).
Binding of erythropoietin and epoetin alfa to EPO-R leads to cellular internalization, which involves the degradation of the ligand. Erythropoietin and epoetin alfa may also be degraded by the reticuloendothelial scavenging pathway or lymphatic system.
**Healthy volunteers:*
The half life is approximately 4 hours in healthy volunteers receiving an intravenous injection. A half-life of approximately 6 hours has been reported in children. **Adult and paediatric patients with CRF:*
The elimination half life following intravenous administration ranges from 4 to 13 hours, which is about 20% longer in CRF patients than that in healthy subjects. The half life is reported to be similar between adult patients receiving or not receiving dialysis. **Cancer patients receiving cyclic chemotherapy:*
Following subcutaneous administration, the average half life is 40 hours with range of 16 to 67 hours.
Erythropoietin or exogenous epoetin alfa binds to the erythropoietin receptor (EPO-R) and activates intracellular signal transduction pathways. The affinity (Kd) of EPO for its receptor on human cells is 100 to 200 pM. Upon binding to EPO-R on the surface of erythroid progenitor cells, a conformational change is induced which brings EPO-R-associated Janus family tyrosine protein kinase 2 (JAK2) molecules into close proximity. JAK2 molecules are subsequently activated via phosphorylation, then phosphorylate tyrosine residues in the cytoplasmic domain of the EPO-R that serve as docking sites for Src homology 2-domain-containing intracellular signaling proteins. The signalling proteins include STAT5 that once phosphorylated by JAK2, dissociates from the EPO-R, dimerizes, and translocates to the nucleus where they serve as transcription factors to activate target genes involved in cell division or differentiation, including the apoptosis inhibitor Bcl-x. The inhibition of apoptosis by the EPO-activated JAK2/STAT5/Bcl-x pathway is critical in erythroid differentiation. Via JAK2-mediated tyrosine phosphorylation, erythropoietin and epoetin alfa also activates other intracellular proteins involved in erythroid cell proliferation and survival, such as Shc , phosphatidylinositol 3-kinase (PI3K), and phospholipase C-1.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37106
Submission : 2022-09-23
Status : Active
Type : II

Certificate Number : CEP 2022-380 - Rev 00
Issue Date : 2024-02-05
Type : Chemical
Substance Number : 2232
Status : Valid

Date of Issue : 2025-02-27
Valid Till : 2028-03-17
Written Confirmation Number : WC-0004
Address of the Firm :

NDC Package Code : 49716-333
Start Marketing Date : 2023-10-15
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20358
Submission : 2007-03-20
Status : Active
Type : II

Registration Number : 222MF10263
Registrant's Address : 8-2-337, Road No. 3, Banjara Hills, Hyderabad 500 034, TELANGANA, INDIA
Initial Date of Registration : 2010-11-29
Latest Date of Registration :

Date of Issue : 2025-06-20
Valid Till : 2028-07-07
Written Confirmation Number : WC-0039
Address of the Firm :

NDC Package Code : 71796-042
Start Marketing Date : 2021-12-03
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Sangjin Trading Co., Ltd.
Registration Date : 2022-06-14
Registration Number : 20220614-122-G-166-39
Manufacturer Name : Dr. Reddy's Laboratories Ltd.
Manufacturer Address : CTO-Unit-V ,Peddadevulapally Village,Tripuraram Mandal, Nalgonda District, Telangana - 508207,INDIA

| Available Reg Filing : ASMF |

 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.

Date of Issue : 2024-08-16
Valid Till : 2027-02-26
Written Confirmation Number : WC-0494
Address of the Firm :
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37106
Submission : 2022-09-23
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : Complete
Rev. Date : 2022-04-20
Pay. Date : 2022-03-31
DMF Number : 36387
Submission : 2021-12-07
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20358
Submission : 2007-03-20
Status : Active
Type : II
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36088
Submission : 2021-07-12
Status : Active
Type : II
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
GDUFA
DMF Review : Complete
Rev. Date : 2020-10-09
Pay. Date : 2020-09-08
DMF Number : 22711
Submission : 2009-04-02
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : Complete
Rev. Date : 2014-02-14
Pay. Date : 2013-08-21
DMF Number : 25654
Submission : 2012-01-03
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16996
Submission : 2003-12-03
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17460
Submission : 2004-06-10
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16526
Submission : 2003-04-05
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16311
Submission : 2002-12-16
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : IOL Chemicals and Pharmaceuticals Limited, with over three decades of experience, is an innovation-driven company specializing in bulk drugs, intermediates, and specialty chemicals...
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
About the Company : Nuray Chemicals Pvt Ltd, established in 2012 near Chennai, is an API manufacturer for highly regulated markets. Its state-of-the-art R&D facility specializes in synthesizing NCEs, ...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
About the Company : Gonane Pharma is a contract pharmaceutical company based in Gujarat, India, specializing in the manufacturing and marketing of corticosteroids, hormones, antivirals, and oncology p...
About the Company : Jai Radhe Sales, founded in 1999, is a global distributor specializing in high-quality pharmaceutical ingredients from India. It offers complete sourcing solutions, technical and r...
About the Company : HRV Pharma is a global manufacturer, seller, and exporter of APIs, intermediates, pellets, food-grade chemicals, food additives, and food ingredients. The company provides sourcing...
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
About the Company : Octavius Pharma is a global leader in Directly Compressible Granules with over 45 years of expertise in formulation development, manufacturing, and commercialization. Our portfolio...
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
About the Company : Tagoor Laboratories, established in 2018 and part of the Tagoor Group, provides APIs, advanced intermediates, and key starting materials for critical and high-growth therapeutic ar...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
About the Company : Zeon Pharma Industries India Pvt. Ltd. is an ISO 9001:2015, cGMP, and WHO-GMP certified company with a dedicated manufacturing facility for Bulk Drugs (APIs), phytochemicals, herba...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
34
PharmaCompass offers a list of Losartan Potassium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Losartan Potassium manufacturer or Losartan Potassium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Losartan Potassium manufacturer or Losartan Potassium supplier.
PharmaCompass also assists you with knowing the Losartan Potassium API Price utilized in the formulation of products. Losartan Potassium API Price is not always fixed or binding as the Losartan Potassium Price is obtained through a variety of data sources. The Losartan Potassium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Losartan Potassium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Losartan Potassium, including repackagers and relabelers. The FDA regulates Losartan Potassium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Losartan Potassium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Losartan Potassium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Losartan Potassium supplier is an individual or a company that provides Losartan Potassium active pharmaceutical ingredient (API) or Losartan Potassium finished formulations upon request. The Losartan Potassium suppliers may include Losartan Potassium API manufacturers, exporters, distributors and traders.
click here to find a list of Losartan Potassium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Losartan Potassium DMF (Drug Master File) is a document detailing the whole manufacturing process of Losartan Potassium active pharmaceutical ingredient (API) in detail. Different forms of Losartan Potassium DMFs exist exist since differing nations have different regulations, such as Losartan Potassium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Losartan Potassium DMF submitted to regulatory agencies in the US is known as a USDMF. Losartan Potassium USDMF includes data on Losartan Potassium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Losartan Potassium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Losartan Potassium suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Losartan Potassium Drug Master File in Japan (Losartan Potassium JDMF) empowers Losartan Potassium API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Losartan Potassium JDMF during the approval evaluation for pharmaceutical products. At the time of Losartan Potassium JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Losartan Potassium suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Losartan Potassium Drug Master File in Korea (Losartan Potassium KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Losartan Potassium. The MFDS reviews the Losartan Potassium KDMF as part of the drug registration process and uses the information provided in the Losartan Potassium KDMF to evaluate the safety and efficacy of the drug.
After submitting a Losartan Potassium KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Losartan Potassium API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Losartan Potassium suppliers with KDMF on PharmaCompass.
A Losartan Potassium CEP of the European Pharmacopoeia monograph is often referred to as a Losartan Potassium Certificate of Suitability (COS). The purpose of a Losartan Potassium CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Losartan Potassium EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Losartan Potassium to their clients by showing that a Losartan Potassium CEP has been issued for it. The manufacturer submits a Losartan Potassium CEP (COS) as part of the market authorization procedure, and it takes on the role of a Losartan Potassium CEP holder for the record. Additionally, the data presented in the Losartan Potassium CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Losartan Potassium DMF.
A Losartan Potassium CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Losartan Potassium CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Losartan Potassium suppliers with CEP (COS) on PharmaCompass.
A Losartan Potassium written confirmation (Losartan Potassium WC) is an official document issued by a regulatory agency to a Losartan Potassium manufacturer, verifying that the manufacturing facility of a Losartan Potassium active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Losartan Potassium APIs or Losartan Potassium finished pharmaceutical products to another nation, regulatory agencies frequently require a Losartan Potassium WC (written confirmation) as part of the regulatory process.
click here to find a list of Losartan Potassium suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Losartan Potassium as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Losartan Potassium API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Losartan Potassium as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Losartan Potassium and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Losartan Potassium NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Losartan Potassium suppliers with NDC on PharmaCompass.
Losartan Potassium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Losartan Potassium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Losartan Potassium GMP manufacturer or Losartan Potassium GMP API supplier for your needs.
A Losartan Potassium CoA (Certificate of Analysis) is a formal document that attests to Losartan Potassium's compliance with Losartan Potassium specifications and serves as a tool for batch-level quality control.
Losartan Potassium CoA mostly includes findings from lab analyses of a specific batch. For each Losartan Potassium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Losartan Potassium may be tested according to a variety of international standards, such as European Pharmacopoeia (Losartan Potassium EP), Losartan Potassium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Losartan Potassium USP).

