


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
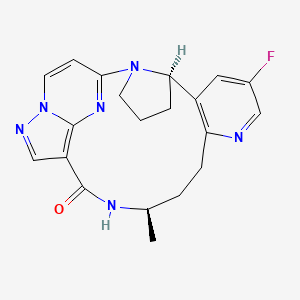

1. 9-fluoro-15-methyl-2,11,16,20,21,24-hexaazapentacyclo(16.5.2.02,6.07,12.021,25)pentacosa-1(24),7,9,11,18(25),19,22-heptaen-17-one
2. Loxo-195
1. Loxo-195
2. 2097002-61-2
3. Loxo195
4. (6r,15r)-9-fluoro-15-methyl-2,11,16,20,21,24-hexazapentacyclo[16.5.2.02,6.07,12.021,25]pentacosa-1(24),7(12),8,10,18(25),19,22-heptaen-17-one
5. 0j45910s3x
6. Unii-0j45910s3x
7. Selitrectinib [inn]
8. Loxo 195(selitrectinib)
9. Selitrectinib [who-dd]
10. Chembl4297627
11. Schembl18823882
12. Gtpl10314
13. Selitrectinib Pound Loxo-195)
14. Ex-a1873
15. Xid00261
16. Bdbm50507492
17. Nsc809970
18. S8636
19. Akos037648883
20. Db14896
21. Nsc-809970
22. Bay 2731954
23. Bay-2731954
24. (13e,14e,22r,6r)-35-fluoro-6-methyl-7-aza-1(5,3)-pyrazolo[1,5-a]pyrimidina-3(3,2)-pyridina-2(1,2)-pyrrolidinacyclooctaphan-8-one
25. Ac-32662
26. Bs-15941
27. Example 28 [wo2017075107a1]
28. Hy-101977
29. Cs-0022378
30. A934608
31. (13e,14e,22r,6r)-35-fluoro-6-methyl-7-aza-1(5,3)pyrazolo[1,5-a]pyrimidina-3(3,2)pyridina-2(1,2)pyrrolidinacyclooctaphan-8-one
32. (22r,6r)-35-fluoro-6-methyl-1(5,3)-pyrazolo(1,5-a)pyrimidina-3(3,2)-pyridina-2(1,2)-pyrrolidinacyclooctaphan-8-one
33. 12h-15,17-ethenopyrazolo[3,4-d]pyrido[2,3-k]pyrrolo[2,1-m][1,3,7]triazacyclotridecin-12-one, 5-fluoro-1,2,3,3a,8,9,10,11-octahydro-10-methyl-, (3ar,10r)-
34. 12h-5,7-ethenopyrazolo(3,4-d)pyrido(2,3-k)pyrrolo(2,1-m)(1,3,7)triazacyclotridecin-12-one, 5-fluoro-1,2,3,3a,8,9,10,11-octahydro-10-methyl-, (3ar,10r)-
35. Cas Registry Number 2097002-61-2 ~1~1 C20 H21 F N6 O 10h-5,7-ethenopyrazolo(3,4-d)pyrido(2,3-k)pyrrolo(2,1-m)(1,3,7)triazacyclotridecin-10-one, 17-fluoro-1,2,3,11,12,13,14,18b-octahydro-12-methyl-, (12r,18br)-
| Molecular Weight | 380.4 g/mol |
|---|---|
| Molecular Formula | C20H21FN6O |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 380.17608748 g/mol |
| Monoisotopic Mass | 380.17608748 g/mol |
| Topological Polar Surface Area | 75.4 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 593 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)


