

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
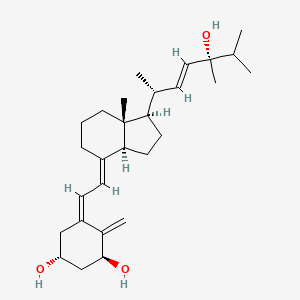

1. 1,24(s)-dihydroxyvitamin D2
2. 1,24-dihydroxyvitamin D2
3. 1alpha,24(s)(oh)2d2
4. 1alpha,24(s)-dihydroxyvitamin D2
5. Lr-103
1. Lr-103
2. 156316-85-7
3. 2900i92z36
4. (24s)-1alpha,24-dihydroxyvitamin D2 / (24s)-1alpha,24-dihydroxyergocalciferol
5. (5z,7e,22e)-(1s,3r,24s)-9,10-seco-5,7,10(19),22-ergostatetraene-1,3,24-triol
6. Unii-2900i92z36
7. Lmst03010062
8. 1alpha,24(s)-dihydroxyergocalciferol
9. Schembl3026089
10. Lr 103 [who-dd]
11. Dtxsid801015488
12. Zinc4791507
13. Db06117
14. (1alpha,3beta,5z,7e,22e,24s)-9,10-secoergosta-5,7,10(19),22-tetraene-1,3,24-triol
15. 1.alpha.,24s-dihydroxyvitamin D2
16. 1.alpha.,24(s)-dihydroxyergocalciferol
17. Q27254357
18. (1.alpha.,3.beta.,5z,7e,22e,24s)-9,10-secoergosta-5,7,10(19),22-tetraene-1,3,24-triol
19. 9,10-secoergosta-5,7,10(19),22-tetraene-1,3,24-triol,(1.alpha.,3.beta.,5z,7e,22e)-
| Molecular Weight | 428.6 g/mol |
|---|---|
| Molecular Formula | C28H44O3 |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 428.32904526 g/mol |
| Monoisotopic Mass | 428.32904526 g/mol |
| Topological Polar Surface Area | 60.7 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 760 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in parathyroid disorders.
LR-103 is effective in normalizing serum calcium and PTH levels without causing hypercalcemia. Importantly, LR-103 also normalized bone abnormalities consequent to SHPT and active vitamin D deficiency.
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
