
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
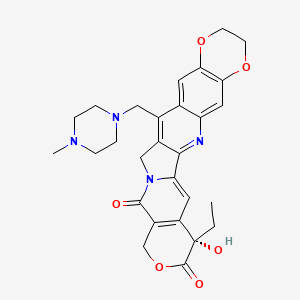

1. 7-(4-methylpiperazinomethylene)-10,11-ethylenedioxy-20(s)-camptothecin
2. Gg-211
3. Gg211
4. Gi 147211
5. Gi-147211
6. Gi147211
7. Gl 147211c
8. Gl-147211c
9. Gl147211c
10. Nx 211
11. Nx-211
12. Nx211 Cpd
13. Osi 211
14. Osi-211
15. Osi211
1. 149882-10-0
2. Osi-211
3. Lurtotecan [inn]
4. 4j1l80t08i
5. Gg-211
6. Nx-211
7. Gi-147211
8. Gg 211; Gi 147211; Lurtotecan; Nx 211; Osi 211
9. Chembl341028
10. (s)-8-ethyl-8-hydroxy-15-((4-methylpiperazin-1-yl)methyl)-11,14-dihydro-2h-[1,4]dioxino[2,3-g]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-9,12(3h,8h)-dione
11. Osi 211
12. Gg 211
13. Nx 211
14. Gi 147211
15. Unii-4j1l80t08i
16. Nx211 Cpd
17. Lurtotecan [who-dd]
18. Schembl19208
19. Chembl305666
20. Osi211
21. Dtxsid30164422
22. Gg211
23. Gl147211c
24. Gl 147211c
25. Bdbm50036130
26. Gw-211
27. Zinc22010625
28. Db12222
29. Gl-147211c
30. 11h-1,4-dioxino(2,3-g)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-9,12(8h,14h)-dione, 8-ethyl-2,3-dihydro-8-hydroxy-15-((4-methyl-1-piperazinyl)methyl)-, (8s)-
31. Gi147211
32. Hy-13670
33. Cs-0007547
34. A936765
35. Q6704977
36. D-myo-inositol2,4,5-trisphosphate,hexaammoniumsalt
37. 7-(4-methylpiperazinomethylene)-10,11-ethylenedioxy-20(s)-camptothecin
38. (18s)-18-ethyl-18-hydroxy-2-[(4-methylpiperazin-1-yl)methyl]-6,9,20-trioxa-13,24-diazahexacyclo[12.11.0.03,12.05,10.015,24.017,22]pentacosa-1,3,5(10),11,13,15,17(22)-heptaene-19,23-dione
39. (8s)-8-ethyl-2,3-dihydro-8-hydroxy-15-((4-methyl-1-piperazinyl)methyl)-11h-p-dioxino(2,3-g)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-9,12(8h,14h)-dione
40. (s)-8-ethyl-8-hydroxy-15-((4-methylpiperazin-1-yl)methyl)-2,3,11,14-tetrahydro-12h-[1,4]dioxino[2,3-g]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-9,12(8h)-dione
41. 11h-1,4-dioxino(2,3-g)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-9,12(8h,14h)-dione, 8-ethyl-2,3-dihydro-8-hydroxy-15-((4-methyl-1-piperazinyl)methyl)-, (s)
42. 8-ethyl-8-hydroxy-15-(4-methylhexahydro-1-pyrazinylmethyl)-(8s)-2,3,8,9,12,14-hexahydro-11h-[1,4]dioxino[2,3-g]pyrano[3'',4'':6,7]indolizino[1,2-b]quinoline-9,12-dione With Trifluoroaceticacid
| Molecular Weight | 518.6 g/mol |
|---|---|
| Molecular Formula | C28H30N4O6 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Exact Mass | 518.21653469 g/mol |
| Monoisotopic Mass | 518.21653469 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 1070 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?