
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
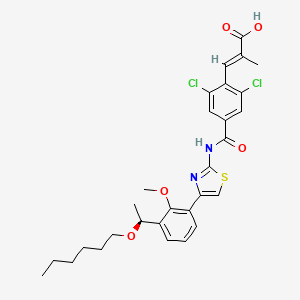

1. Mulpleta
1. 1110766-97-6
2. Mulpleta
3. S-888711
4. 6ll5jfu42f
5. Rsc888711
6. (e)-3-[2,6-dichloro-4-[[4-[3-[(1s)-1-hexoxyethyl]-2-methoxyphenyl]-1,3-thiazol-2-yl]carbamoyl]phenyl]-2-methylprop-2-enoic Acid
7. (s,e)-3-(2,6-dichloro-4-((4-(3-(1-(hexyloxy)ethyl)-2-methoxyphenyl)thiazol-2-yl)carbamoyl)phenyl)-2-methylacrylic Acid
8. (2e)-3-(2,6-dichloro-4-((4-(3-((1s)-1-(hexyloxy)ethyl)-2-methoxyphenyl)-1,3-thiazol-2-yl)carbamoyl)phenyl)-2-methylprop-2-enoic Acid
9. Lusutrombopag [inn]
10. Unii-6ll5jfu42f
11. Lusutrombopag [usan:inn]
12. Mulpleta (tn)
13. 2-propenoic Acid, 3-(2,6-dichloro-4-(((4-(3-((1s)-1-(hexyloxy)ethyl)-2-methoxyphenyl)-2-thiazolyl)amino)carbonyl)phenyl)-2-methyl-, (2e)-
14. S 888711
15. Lusutrombopags-888711
16. Lusutrombopag [mi]
17. Lusutrombopag [jan]
18. Lusutrombopag [usan]
19. Lusutrombopag [who-dd]
20. Schembl3062080
21. Schembl3062084
22. Chembl2107831
23. Lusutrombopag (jan/usan/inn)
24. Gtpl10032
25. Chebi:136051
26. Dtxsid701027951
27. Lusutrombopag [orange Book]
28. 2-propenoic Acid, 3-[2,6-dichloro-4-[[[4-[3-[(1s)-1-(hexyloxy)ethyl]-2-methoxyphenyl]-2-thiazolyl]amino]carbonyl]phenyl]-2-methyl-, (2e)-
29. Ex-a1290
30. Mfcd28502075
31. S6988
32. Zinc84759273
33. Cs-6137
34. Db13125
35. Ncgc00522464-01
36. (2e)-3-(2,6-dichloro-4-((4-(3-((1s)-1-(hexyloxy)ethyl)- 2-methoxyphenyl)-1,3-thiazol-2-yl)carbamoyl)phenyl)-2-methylprop-2-enoic Acid
37. Ac-30601
38. As-52368
39. Hy-19883
40. J3.505.027b
41. D10476
42. A927042
43. S888711
44. Q27265116
45. (e)-3-[2,6-dichloro-4-[4-[3-[(s)-1-hexyloxyethyl]-2-methoxyphenyl]thiazol-2-ylcarbamoyl]phenyl]-2-methylacrylic Acid
| Molecular Weight | 591.5 g/mol |
|---|---|
| Molecular Formula | C29H32Cl2N2O5S |
| XLogP3 | 7.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 13 |
| Exact Mass | 590.1408987 g/mol |
| Monoisotopic Mass | 590.1408987 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 822 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Lusutrombopag is indicated for the treatment of thrombocytopenia in adults with chronic liver disease who are scheduled to undergo a medical or dental procedure.
FDA Label
Mulpleo is indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease undergoing invasive procedures
Treatment of thrombocytopenia secondary to liver disease
The AUC of lusutrombopag was found to correlate the increased platelet counts. Following administration of 3 mg daily dose in patients with chronic liver disease and thrombocytopenia, the mean (standard deviation) maximum platelet count in patients (N=74) without platelet transfusion was 86.9 (27.2) 10^9/L, and the median time to reach the maximum platelet count was 12.0 (5 to 35) days. Lusutrombopag was not shown to induce any clinically significant QTc prolongation at a dose 8 times the recommended dosage.
B02BX
B - Blood and blood forming organs
B02 - Antihemorrhagics
B02B - Vitamin k and other hemostatics
B02BX - Other systemic hemostatics
B02BX07 - Lusutrombopag
Absorption
Lusutrombopag is rapidly absorbed following oral administration. It exhibited a doseproportional pharmacokinetic profile over the single dose range of 1 mg to 50 mg, which was similar in both healthy subjects and those with chronic liver disease. A geometric mean (%CV) maximal concentration (Cmax) and area under the curve (AUC) in healthy subjects receiving 3 mg of lusutrombopag were 111 (20.4) ng/mL and 2931 (23.4) ng.hr/mL. The accumulation ratios of Cmax and AUC were approximately 2 with oncedaily multipledose administration, and steadystate plasma lusutrombopag concentrations were achieved after Day 5. The time to reach peak plasma concentrations (Tmax) were approximately 6 to 8 hours after oral administration in patients with chronic liver disease. Food consumption is not reported to affect the absorption and bioavailability of lusutrombopag.
Route of Elimination
About 1% of the administered dose of lusutrombopag undergoes urinary excretion. Fecal excretion accounted for 83% of the total dose, where 16% of the dose was excreted as unchanged parent compound.
Volume of Distribution
The mean (%CV) lusutrombopag apparent volume of distribution in healthy adult subjects was 39.5 (23.5) L.
Clearance
The approximate mean (%CV) clearance of lusutrombopag in patients with chronic liver disease is estimated to be 1.1 (36.1) L/hr.
CYP4 enzymes predominantly contribute to the metabolism of lusutrombopag, especially CYP4A11. Lusutrombopag is reported to mainly undergo - and -oxidation, as well as glucuronidation.
In healthy adult subjects, the terminal elimination halflife (t1/2) was approximately 27 hours.
Lusutrombopag mimics the biological actions of endogenous thrombopoietin (TPO) by acting as an agonist for the thrombopoietin receptor (TPOR) expressed on megakaryocytes. It binds to the transmembrane domain of the receptor and induces thrombocytopoiesis by targeting the same signal transduction system as that of endogenous TPO, which involves the activation of JAK and STAT pathways. It stimulates the proliferation and differentiation of bone marrow progenitor cells into megakaryocytes, which undergoes maturation to act as precursor cells for platelets. A single megakaryocyte produces and releases thousands of platelets upon maturation and series of remodeling events. Lusutrombopag displays high specificity towards human TPORs when compared to murine TPORs. Lusutrombopag may affect other hematopoietic lineages as well, including erythroid, granulocytic and lymphoid lineages. One case of increased leukocyte and erythrocyte counts that prolonged for over 120 days was reported following administration in a patient with liver cirrhosis (LC) due to hepatitis C virus.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
35
PharmaCompass offers a list of Lusutrombopag API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lusutrombopag manufacturer or Lusutrombopag supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lusutrombopag manufacturer or Lusutrombopag supplier.
PharmaCompass also assists you with knowing the Lusutrombopag API Price utilized in the formulation of products. Lusutrombopag API Price is not always fixed or binding as the Lusutrombopag Price is obtained through a variety of data sources. The Lusutrombopag Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lusutrombopag manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lusutrombopag, including repackagers and relabelers. The FDA regulates Lusutrombopag manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lusutrombopag API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lusutrombopag manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lusutrombopag supplier is an individual or a company that provides Lusutrombopag active pharmaceutical ingredient (API) or Lusutrombopag finished formulations upon request. The Lusutrombopag suppliers may include Lusutrombopag API manufacturers, exporters, distributors and traders.
click here to find a list of Lusutrombopag suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lusutrombopag as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lusutrombopag API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lusutrombopag as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lusutrombopag and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lusutrombopag NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lusutrombopag suppliers with NDC on PharmaCompass.
Lusutrombopag Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lusutrombopag GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lusutrombopag GMP manufacturer or Lusutrombopag GMP API supplier for your needs.
A Lusutrombopag CoA (Certificate of Analysis) is a formal document that attests to Lusutrombopag's compliance with Lusutrombopag specifications and serves as a tool for batch-level quality control.
Lusutrombopag CoA mostly includes findings from lab analyses of a specific batch. For each Lusutrombopag CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lusutrombopag may be tested according to a variety of international standards, such as European Pharmacopoeia (Lusutrombopag EP), Lusutrombopag JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lusutrombopag USP).
