
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers
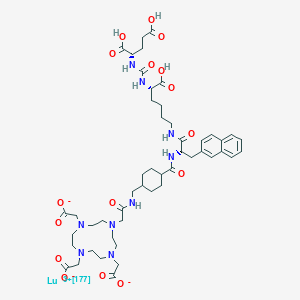

1. 177lu-617-prostate-specific Membrane Antigen Ligand
2. 177lu-psma-617
1. 177lu-psma-617
2. G6uf363ecx
3. Vipivotide Tetraxetan Lu-177
4. 177lu-labeled Psma-617
5. Lutetium Lu-177 Vipivotide Tetraxetan
6. Vipivotide Tetraxetan Lutetium Lu-177
7. Lutetium (177lu) Vipivotide Tetraxetan
8. Lutetium (177lu) Vipivotide Tetraxetan [inn]
9. Lutetium Lu 177 Vipivotide Tetraxetan [usan]
10. Pluvicto
11. Unii-g6uf363ecx
12. Who 11429
13. Lutetium (177lu) Vipivotide Tetraxetan [who-dd]
14. 1703749-62-5
15. 1983157-55-6
16. Lutetate(3-)-177lu, (n-((((1s)-1-carboxy-5-(((2s)-3-(2-naphthalenyl)-1-oxo-2-(((trans-4-(((2-(4,7,10-tris((carboxy-.kappa.o)methyl)-1,4,7,10-tetraazacyclododec-1-yl-.kappa.n1,.kappa.n4,.kappa.n7,.kappa.n10)acetyl-.kappa.o)amino)methyl)cyclohexyl)carbonyl)amino)propyl)amino)pentyl)amino)carbonyl)-l-glutamato(6-))-, Hydrogen (1:3)
17. Lutetate(3-)-177lu, (n2-((((1s)-1,3-dicarboxypropyl)amino)carbonyl)-n6-(3-(2-naphthalenyl)-n-((trans-4-(((2-(4,7,10-tris((carboxy-.kappa.o)methyl)-1,4,7,10-tetraazacyclododec-1-yl-.kappa.n1,.kappa.n4,.kappa.n7,.kappa.n10)acetyl-.kappa.o)amino)methyl)cyclohexyl)carbonyl)-l-alanyl)-l-lysinato(6-))-, Hydrogen (1:3
| Molecular Weight | 1216.1 g/mol |
|---|---|
| Molecular Formula | C49H68LuN9O16 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 24 |
| Exact Mass | 1215.42217 g/mol |
| Monoisotopic Mass | 1215.42217 g/mol |
| Topological Polar Surface Area | 374 Ų |
| Heavy Atom Count | 75 |
| Formal Charge | 0 |
| Complexity | 1860 |
| Isotope Atom Count | 1 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of PSMA-expressing prostate cancer
Radiopharmaceuticals
Compounds that are used in medicine as sources of radiation for radiotherapy and for diagnostic purposes. They have numerous uses in research and industry. (Martindale, The Extra Pharmacopoeia, 30th ed, p1161) (See all compounds classified as Radiopharmaceuticals.)
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
17
PharmaCompass offers a list of Lutetium-177 Vipivotide Tetraxetan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lutetium-177 Vipivotide Tetraxetan manufacturer or Lutetium-177 Vipivotide Tetraxetan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lutetium-177 Vipivotide Tetraxetan manufacturer or Lutetium-177 Vipivotide Tetraxetan supplier.
PharmaCompass also assists you with knowing the Lutetium-177 Vipivotide Tetraxetan API Price utilized in the formulation of products. Lutetium-177 Vipivotide Tetraxetan API Price is not always fixed or binding as the Lutetium-177 Vipivotide Tetraxetan Price is obtained through a variety of data sources. The Lutetium-177 Vipivotide Tetraxetan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lutetium Lu 177 Vipivotide Tetraxetan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lutetium Lu 177 Vipivotide Tetraxetan, including repackagers and relabelers. The FDA regulates Lutetium Lu 177 Vipivotide Tetraxetan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lutetium Lu 177 Vipivotide Tetraxetan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Lutetium Lu 177 Vipivotide Tetraxetan supplier is an individual or a company that provides Lutetium Lu 177 Vipivotide Tetraxetan active pharmaceutical ingredient (API) or Lutetium Lu 177 Vipivotide Tetraxetan finished formulations upon request. The Lutetium Lu 177 Vipivotide Tetraxetan suppliers may include Lutetium Lu 177 Vipivotide Tetraxetan API manufacturers, exporters, distributors and traders.
click here to find a list of Lutetium Lu 177 Vipivotide Tetraxetan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lutetium Lu 177 Vipivotide Tetraxetan DMF (Drug Master File) is a document detailing the whole manufacturing process of Lutetium Lu 177 Vipivotide Tetraxetan active pharmaceutical ingredient (API) in detail. Different forms of Lutetium Lu 177 Vipivotide Tetraxetan DMFs exist exist since differing nations have different regulations, such as Lutetium Lu 177 Vipivotide Tetraxetan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lutetium Lu 177 Vipivotide Tetraxetan DMF submitted to regulatory agencies in the US is known as a USDMF. Lutetium Lu 177 Vipivotide Tetraxetan USDMF includes data on Lutetium Lu 177 Vipivotide Tetraxetan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lutetium Lu 177 Vipivotide Tetraxetan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lutetium Lu 177 Vipivotide Tetraxetan suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lutetium Lu 177 Vipivotide Tetraxetan Drug Master File in Japan (Lutetium Lu 177 Vipivotide Tetraxetan JDMF) empowers Lutetium Lu 177 Vipivotide Tetraxetan API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lutetium Lu 177 Vipivotide Tetraxetan JDMF during the approval evaluation for pharmaceutical products. At the time of Lutetium Lu 177 Vipivotide Tetraxetan JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lutetium Lu 177 Vipivotide Tetraxetan suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lutetium Lu 177 Vipivotide Tetraxetan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lutetium Lu 177 Vipivotide Tetraxetan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lutetium Lu 177 Vipivotide Tetraxetan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lutetium Lu 177 Vipivotide Tetraxetan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lutetium Lu 177 Vipivotide Tetraxetan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lutetium Lu 177 Vipivotide Tetraxetan suppliers with NDC on PharmaCompass.
Lutetium Lu 177 Vipivotide Tetraxetan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lutetium Lu 177 Vipivotide Tetraxetan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lutetium Lu 177 Vipivotide Tetraxetan GMP manufacturer or Lutetium Lu 177 Vipivotide Tetraxetan GMP API supplier for your needs.
A Lutetium Lu 177 Vipivotide Tetraxetan CoA (Certificate of Analysis) is a formal document that attests to Lutetium Lu 177 Vipivotide Tetraxetan's compliance with Lutetium Lu 177 Vipivotide Tetraxetan specifications and serves as a tool for batch-level quality control.
Lutetium Lu 177 Vipivotide Tetraxetan CoA mostly includes findings from lab analyses of a specific batch. For each Lutetium Lu 177 Vipivotide Tetraxetan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lutetium Lu 177 Vipivotide Tetraxetan may be tested according to a variety of international standards, such as European Pharmacopoeia (Lutetium Lu 177 Vipivotide Tetraxetan EP), Lutetium Lu 177 Vipivotide Tetraxetan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lutetium Lu 177 Vipivotide Tetraxetan USP).
