
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Calcium (hydroxy-1-malate) Hexahydrate
2. Malate
3. Malic Acid, (r)-isomer
4. Malic Acid, Calcium Salt, (1:1), (s)-isomer
5. Malic Acid, Disodium Salt
6. Malic Acid, Disodium Salt, (r)-isomer
7. Malic Acid, Disodium Salt, (s)-isomer
8. Malic Acid, Magnesium Salt (2:1)
9. Malic Acid, Monopotassium Salt, (+-)-isomer
10. Malic Acid, Potassium Salt, (r)-isomer
11. Malic Acid, Sodium Salt, (+-)-isomer
1. Dl-malic Acid
2. 6915-15-7
3. 2-hydroxybutanedioic Acid
4. 2-hydroxysuccinic Acid
5. 617-48-1
6. Malate
7. Butanedioic Acid, Hydroxy-
8. Hydroxysuccinic Acid
9. Malic Acid, Dl-
10. Kyselina Jablecna
11. Hydroxybutanedioic Acid
12. Pomalus Acid
13. Deoxytetraric Acid
14. Dl-hydroxybutanedioic Acid
15. Hydroxybutandisaeure
16. Alpha-hydroxysuccinic Acid
17. Musashi-no-ringosan
18. Caswell No. 537
19. Dl-2-hydroxybutanedioic Acid
20. Fda 2018
21. Monohydroxybernsteinsaeure
22. Succinic Acid, Hydroxy-
23. R,s(+-)-malic Acid
24. Kyselina Jablecna [czech]
25. Malic Acid [nf]
26. Fema No. 2655
27. 2-hydroxyethane-1,2-dicarboxylic Acid
28. Pomalous Acid
29. Kyselina Hydroxybutandiova [czech]
30. D,l-malic Acid
31. Epa Pesticide Chemical Code 051101
32. Ai3-06292
33. (+/-)-malic Acid
34. Malic Acid, L-
35. Nsc-25941
36. E296
37. Butanedioic Acid, Hydroxy-, (s)-
38. Mls000084707
39. 817l1n4ckp
40. Chebi:6650
41. Ins No.296
42. (+-)-1-hydroxy-1,2-ethanedicarboxylic Acid
43. Ins No. 296
44. Ins-296
45. Nsc25941
46. Malic Acid (nf)
47. Smr000019054
48. Dl-apple Acid
49. E-296
50. Dsstox_cid_7640
51. (r)-hydroxybutanedioic Acid
52. (s)-hydroxybutanedioic Acid
53. Dsstox_rid_78538
54. Dsstox_gsid_27640
55. (+-)-malic Acid
56. R-malic Acid
57. Malicum Acidum
58. Fema Number 2655
59. Butanedioic Acid, 2-hydroxy-, (2s)-
60. Cas-6915-15-7
61. Ccris 2950
62. Ccris 6567
63. L-(-)-malicacid
64. Hsdb 1202
65. Dl-hydroxysuccinic Acid
66. Kyselina Hydroxybutandiova
67. Einecs 210-514-9
68. Einecs 230-022-8
69. Nsc 25941
70. Hydroxybutanedioic Acid, (-)-
71. (+-)-hydroxysuccinic Acid
72. Unii-817l1n4ckp
73. Aepfelsaeure
74. Nsc 9232
75. Mfcd00004245
76. Mfcd00064213
77. (+/-)-2-hydroxysuccinic Acid
78. Hydroxybutanedioic Acid, (+-)-
79. H2mal
80. Racemic Malic Acid
81. Mfcd00064212
82. .+-.-malic Acid
83. 143435-96-5
84. Opera_id_805
85. 2-hydroxyl-succinic Acid
86. Dl-malic Acid, 99%
87. Malic Acid [ii]
88. Malic Acid [mi]
89. Malic Acid,(dl)
90. 2-hydroxydicarboxylic Acid
91. Malic Acid [fcc]
92. Schembl856
93. 2-hydroxy-butanedioic Acid
94. Bmse000046
95. Bmse000904
96. Malic Acid [inci]
97. Ec 210-514-9
98. Ec 230-022-8
99. Malic Acid [vandf]
100. Malic Acid-, (l-form)-
101. Dl-malic Acid, >=99%
102. Hyoscyaminehydrobromide
103. Oprea1_130558
104. Oprea1_624131
105. Malic Acid [usp-rs]
106. Malic Acid [who-dd]
107. Butanedioic Acid, 2-hydroxy-
108. Dl-malic Acid-2-[13c]
109. Dl-hydroxysucoinic Acid
110. Butanedioic Acid, (.+-.)-
111. Dl(+/-)-malic Acid
112. Gtpl2480
113. 2-hydroxy-succinic Acid
114. Dl-hyroxybutanedioic Acid
115. Chembl1455497
116. Dtxsid0027640
117. Bdbm92495
118. Malic Acid [ep Monograph]
119. Malic Acid [usp Impurity]
120. Dl-malic Acid, Fcc, >=99%
121. Hms2358h06
122. Hms3371c13
123. Dl-malic Acid, Analytical Standard
124. Hy-y1311
125. Str03457
126. (+/-)-hydroxysuccinic Acid
127. Tox21_201536
128. Tox21_300372
129. S9001
130. Stl283959
131. Hydroxybutanedioic Acid [hsdb]
132. Akos000120085
133. Akos017278471
134. (+/-)-hydroxybutanedioic Acid
135. Am81418
136. Ccg-266122
137. Db12751
138. Dl-malic Acid, Reagentplus(r), 99%
139. Ncgc00043225-02
140. Ncgc00043225-03
141. Ncgc00254259-01
142. Ncgc00259086-01
143. Dl-malic Acid, >=98% (capillary Gc)
144. Hydroxybutanedioic Acid, (+/-)-
145. Sy003313
146. Sy009804
147. Dl-malic Acid, Reagentplus(r), >=99%
148. Db-016133
149. Dl-malic Acid 1000 Microg/ml In Methanol
150. Dl-malic Acid, Usp, 99.0-100.5%
151. Cs-0017784
152. E 296
153. Eu-0067046
154. Ft-0605225
155. Ft-0625484
156. Ft-0625485
157. Ft-0625539
158. Ft-0632189
159. M0020
160. Dl-malic Acid, Saj First Grade, >=99.0%
161. A19426
162. C00711
163. C03668
164. D04843
165. Dl-malic Acid 1000 Microg/ml In Acetonitrile
166. Dl-malic Acid, Vetec(tm) Reagent Grade, 98%
167. M-0825
168. Ab00443952-12
169. Malic Acid, Meets Usp/nf Testing Specifications
170. 4-ethoxyphenyltrans-4-propylcyclohexanecarboxylate
171. L023999
172. Q190143
173. Q-201028
174. 0c9a2dc0-fea2-4864-b98b-0597cdd0ad06
175. F0918-0088
176. Z940713496
177. Malic Acid, United States Pharmacopeia (usp) Reference Standard
178. Malic Acid (constituent Of Cranberry Liquid Preparation) [dsc]
179. Malic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
180. Dl-malic Acid, Meets Analytical Specification Of Fcc, E296, 99-100.5% (alkalimetric)
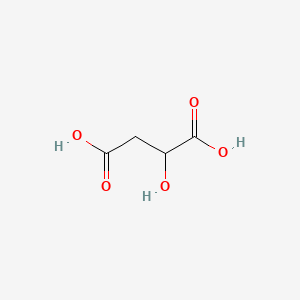
| Molecular Weight | 134.09 g/mol |
|---|---|
| Molecular Formula | C4H6O5 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 134.02152329 g/mol |
| Monoisotopic Mass | 134.02152329 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 129 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EXPL THER An efficcacy and safety test of a tablet containing 200 mg malic acid (and 50 mg magnesium) was conducted using patients with primary fibromyalgia syndrome. In the first part of the test, 24 patients were given three tablets twice daily (bid) for 4 weeks. In the second part, 16 patients started with three tablets bid and increased the dosage every 3 to 5 days as necessary; at month 6, the average dose was 8.8 tablets per day. (For a 50-kg person, ingestion of six tablets would be equivalent to 24 mg of malate/kg of body weight). In the first part of the study, one test patient reported diarrhea, one reported nausea, and one reported dyspepsia. (In the placebo group, two patients reported diarrhea and one reported dyspepsia.) In the second part of the study, five test patients reported diarrhea, one reported nausea, one reported dyspepsia, one reported panic attacks, and one reported dizziness.
Fuime MZ; Int J Toxicol 20 (Suppl 1): 47-55 (2001)
EXPL THER Organic acids in Chinese herbs, the long-neglected components, have been reported to possess antioxidant, anti-inflammatory, and antiplatelet aggregation activities; thus they may have potentially protective effect on ischemic heart disease. Therefore, this study aims to investigate the protective effects of two organic acids, that is, citric acid and L-malic acid, which are the main components of Fructus Choerospondiatis, on myocardial ischemia/reperfusion injury and the underlying mechanisms. In in vivo rat model of myocardial ischemia/reperfusion injury, we found that treatments with citric acid and L-malic acid significantly reduced myocardial infarct size, serum levels of TNF-alpha, and platelet aggregation. In vitro experiments revealed that both citric acid and L-malic acid significantly reduced LDH release, decreased apoptotic rate, downregulated the expression of cleaved caspase-3, and upregulated the expression of phosphorylated Akt in primary neonatal rat cardiomyocytes subjected to hypoxia/reoxygenation injury. These results suggest that both citric acid and L-malic acid have protective effects on myocardial ischemia/reperfusion injury; the underlying mechanism may be related to their anti-inflammatory, antiplatelet aggregation and direct cardiomyocyte protective effects. These results also demonstrate that organic acids, besides flavonoids, may also be the major active ingredient of Fructus Choerospondiatis responsible for its cardioprotective effects and should be attached great importance in the therapy of ischemic heart disease. /L-Malic Acid/
PMID:23737849 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3666396 Tang X et al; Evid Based Complement Alternat Med. (2013) Published online 2013 May 14. doi: 10.1155/2013/820695
EXPL THER Objectives: Assessing the clinical effectiveness of a topical sialogogue on spray (malic acid, 1%) in the treatment of xerostomia induced by antihypertensive drugs. Study Design: This research has been carried out through a randomized double-blind clinical trial. 45 patients suffering from hypertensive drugs-induced xerostomia were divided into 2 groups: the first group (25 patients) received a topical sialogogue on spray (malic acid, 1%) whereas the second group (20 patients) received a placebo. Both of them were administered on demand for 2 weeks. Dry Mouth Questionnaire (DMQ) was used in order to evaluate xerostomia levels before and after product/placebo application. Unstimulated and stimulated salivary flows rates, before and after application, were measured. All the statistical analyses were performed by using SPSS software v17.0. Different DMQ scores at the earliest and final stage of the trial were analysed by using Mann-Whitney U test, whereas Student's T-test was used to analyse salivary flows. Critical p-value was established at p<0.05. Results: DMQ scores increased significantly (clinical recovery) from 1.21 to 3.36 points (p<0.05) after malic acid (1%) application whereas DMQ scores increased from 1.18 to 1.34 points (p>0.05) after placebo application. After two weeks of treatment with malic acid, unstimulated salivary flow increased from 0.17 to 0.242 mL/min whereas the stimulated one increased from 0.66 to 0.92 mL/min (p<0.05). After placebo application unstimulated flow ranged from 0.152 to 0.146 mL/min and stimulated flow increased from 0.67 to 0.70 mL/min (p>0.05). Conclusions: Malic acid 1% spray improved antihypertensive-induced xerostomia and stimulated the production of saliva.
Gomez-Moreno G et al; Med Oral Patol Oral Cir Bucal. 18 (1): e49-e55 (2013)
Fourteen patients, 11 males and 3 females, with various forms of ichthyosiformdermatoses were used to evaluate the therapeutic potential of more than 60 chemicals, including malic acid. Malic acid was dissolved in either water or ethanol and incorporated into a hydrophilic ointment of plain petrolatum. The ointment, containing 5% malic acid (pH not specified), was applied twice daily to the appropriate test site for 2 weeks. Daily to weekly observations were made. Malic acid provided 3+ (disappearance of scales from lesions) or 4+ (restoration to normal looking skin) improvement in all patients except one with epidermolytic hyperkeratosis.
Fuime MZ; Int J Toxicol 20 (Suppl 1): 47-55 (2001)
Upon oral and IP administration of radioactive malic acid to rats, most of the radioactivity was excreted as carbon dioxide.
Fuime MZ; Int J Toxicol 20 (Suppl 1): 47-55 (2001)
Acidulents. Like l-(14)C4 malic acid, dl-(14)C4 malic acid, when admin ip or orally to rats was extensively metabolized; 90-95% of (14)C was excreted through lungs as (14)CO2. ... Metabolized at same rate irrespective of route admin ... . /L- & dl-malic acid/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 246
Malic acid is an intermediate in the citric acid cycle. It is formed from fumaric acid and is oxidized to oxaloacetic acid. It is also metabolized to pyruvic acid by malic enzyme which is present in many biologic systems, including bacteria and plants. L-Malic and dl-malic acid are both rapidly metabolized in the rat. Orally or ip administered l- or dl-malic acid was extensively eliminated as carbon dioxide (83 to 92%). No differences between the two forms were found in the rates (90 to 95% in 24 hr) or routes of excretion.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4942
Malates are normal constituents of the diet of humans and animals and, when ingested, are rapidly and completely metabolized to CO2. /Malates/
EFSA Journal 12 (2): 3563 (2014)
... Both enantiomers of malic acid are readily metabolised by laboratory animals and humans and that there was no reason to distinguish between L-malic acid and DL-malic acid when considering their safe use in food.
EFSA Journal 12 (2): 3563 (2014)
Upon oral and IP administration of radioactive Malic Acid to rats, most of the radioactivity was excreted as carbon dioxide.
Fuime MZ; Int J Toxicol 20 (Suppl 1): 47-55 (2001)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
68
PharmaCompass offers a list of Malic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Malic Acid manufacturer or Malic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Malic Acid manufacturer or Malic Acid supplier.
PharmaCompass also assists you with knowing the Malic Acid API Price utilized in the formulation of products. Malic Acid API Price is not always fixed or binding as the Malic Acid Price is obtained through a variety of data sources. The Malic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Malic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Malic Acid, including repackagers and relabelers. The FDA regulates Malic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Malic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Malic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Malic Acid supplier is an individual or a company that provides Malic Acid active pharmaceutical ingredient (API) or Malic Acid finished formulations upon request. The Malic Acid suppliers may include Malic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Malic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Malic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Malic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Malic Acid GMP manufacturer or Malic Acid GMP API supplier for your needs.
A Malic Acid CoA (Certificate of Analysis) is a formal document that attests to Malic Acid's compliance with Malic Acid specifications and serves as a tool for batch-level quality control.
Malic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Malic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Malic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Malic Acid EP), Malic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Malic Acid USP).
