

Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
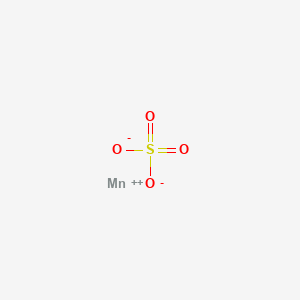

1. Manganese(ii) Sulfate
2. 7785-87-7
3. Manganous Sulfate
4. Manganese(2+) Sulfate
5. Manganese Monosulfate
6. Manganese Sulfate Anhydrous
7. Manganese Sulfate (1:1)
8. Mnso4
9. 10124-55-7
10. Manganese(2+);sulfate
11. Manganese(2+) Sulfate (1:1)
12. Iga15s9h40
13. Sorba-spray Mn
14. Man-gro
15. Sorba-spray Manganese
16. Sulfuric Acid, Manganese Salt
17. Manganese (ii) Sulfate Solution
18. Manganese Sulfate (mnso4)
19. Sulfato De Manganeso
20. Ccris 6916
21. Hsdb 2187
22. Nci C61143
23. Einecs 232-089-9
24. Unii-iga15s9h40
25. Sulfuric Acid, Manganese (ii) Salt (1:1)
26. Manganese(ii) Sulfate (1:1)
27. Einecs 233-342-6
28. Manganese(ii)sulfate
29. Manganese(ii) Sulphate
30. Manganese(2+) Sulphate
31. Ec 232-089-9
32. Manganese Sulfate [mi]
33. Dtxsid9044160
34. Chebi:86360
35. Manganese(ii) Sulfate (mnso4)
36. Manganese (ii )sulfate Anhydrous
37. Manganese Sulfate [who-dd]
38. Manganese Sulphate Anhydrous
39. Manganese Sulfate, Anhydrous
40. Manganese Sulfate (anhydrous)
41. Mangani(ii) Sulfas Anhydricus
42. Akos015904462
43. Manganese (as Sulfate) [vandf]
44. Manganese(2+) Sulphate (1:1)
45. Manganese Sulfate Anhydrous [hsdb]
46. Ft-0628155
47. M3394
48. Q409393
49. J-521672
| Molecular Weight | 151.00 g/mol |
|---|---|
| Molecular Formula | MnO4S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 150.889773 g/mol |
| Monoisotopic Mass | 150.889773 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
MEDICATION (VET): To prevent or treat manganese deficiency.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 937
MEDICATION (VET): NUTRITIONAL FACTOR (ESSENTIAL TRACE ELEMENT IN ALL ANIMALS); PREVENTION OF PEROSIS IN POULTRY.
The Merck Index. 10th ed. Rahway, New Jersey: Merck Co., Inc., 1983., p. 818
MEDICATION: NUTRIENT &/OR DIETARY SUPPLEMENTAL FOOD ADDITIVE
Furia, T.E. (ed.). CRC Handbook of Food Additives. 2nd ed. Cleveland: The Chemical Rubber Co., 1972., p. 383
WHEN LARGE DOSES OF MANGANESE SULFATE ARE INJECTED IV EXCRETION IS ALMOST EXCLUSIVELY IN FECES ... .
Browning, E. Toxicity of Industrial Metals. 2nd ed. New York: Appleton-Century-Crofts, 1969., p. 215
/Manganese sulfate/ is poorly absorbed through lung and gut.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-144

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
