
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
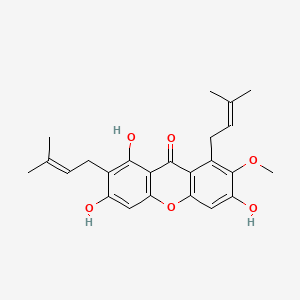

1. 1,3,6,7-tetrahydroxy-2,5-bis(3-methyl-2-butenyl)-9h-xanthen-9-one
2. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2- Butenyl)-9h-xanthen-9-one
3. 7-o-methyl-4-desprenylcostatin
4. 7-o-methyl-gamma-mangostin
5. Alfa-mangostin
6. Alpha-mangosten
7. Gamma-mangostin
8. Mangostin
1. Mangostin
2. 6147-11-1
3. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-9h-xanthen-9-one
4. Mangostine
5. Alpha-mangostin, 95%
6. A-mangostin
7. Nsc-30552
8. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-enyl)xanthen-9-one
9. Nsc27593
10. Nsc30552
11. U6riv93ru1
12. Chembl323197
13. Chebi:67547
14. 1,3,6-trihydroxy-7-methoxy-2,8-diprenylxanthone
15. Tnp00140
16. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-9h-xanthen-9-one
17. Nsc-27593
18. Nsc-139154
19. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3,3-dimethylallyl)xanthone
20. 9h-xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-
21. 1,3,6-trihydroxy-7-methoxy-2,8-di(3-methyl-2-butenyl)xanthone
22. 3,6,8-trihydroxy-2-methoxy-1,7-bis(3-methylbut-2-enyl)xanthen-9-one
23. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-enyl)-9h-xanthen-9-one
24. Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-
25. 7-o-methyl-4-desprenylcostatin
26. 9h-xanthen-9-one,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-
27. Xanthen-9-one,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-
28. .alpha.-mangosten
29. .alpha.-mangostin
30. Mfcd00135200
31. Nsc 27593
32. Nsc 30552
33. Nsc 139154
34. Spectrum_001726
35. Specplus_000574
36. Mangostin [mi]
37. Mangostin [inci]
38. Spectrum2_001620
39. Spectrum3_001297
40. Spectrum4_001911
41. Spectrum5_000622
42. Unii-u6riv93ru1
43. Bspbio_002933
44. Kbiogr_002529
45. Kbioss_002206
46. Bidd:er0576
47. Divk1c_006670
48. Schembl354735
49. Spectrum1504015
50. Spectrum1505128
51. 7-o-methyl-.gamma.-mangostin
52. Spbio_001659
53. Hsdb 8103
54. Kbio1_001614
55. Kbio2_002206
56. Kbio2_004774
57. Kbio2_007342
58. Kbio3_002153
59. Dtxsid00210420
60. Act09245
61. Bcp13253
62. Hy-n0328
63. Zinc5430812
64. Alpha-mangostin, >=98% (hplc)
65. 1,7-bis(3-methylbut-2-enyl)-3,6,8-trihydroxy-2-methoxyxanthen-9-one
66. Bdbm50214969
67. Ccg-36465
68. Nsc139154
69. S3804
70. 9h-xanthen-9-one,1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-
71. Akos015912806
72. Ac-6089
73. Cs-6435
74. Ds-3359
75. Sdccgmls-0066796.p001
76. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)-9h-xanthen-9-one
77. Ncgc00017251-01
78. Ncgc00017251-02
79. Ncgc00017251-03
80. Ncgc00017251-04
81. Ncgc00017251-05
82. Ncgc00095730-01
83. Ncgc00095730-02
84. Ncgc00095730-03
85. Ncgc00178385-01
86. Db-053898
87. Ft-0635997
88. M2793
89. N1590
90. N2590
91. 147m111
92. A833244
93. Q909638
94. Sr-05000002649
95. Q-100010
96. Sr-05000002649-1
97. Brd-k11991978-001-02-6
98. Brd-k11991978-001-03-4
99. 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-enyl)-9-xanthenone
100. 1,3,6-trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2-enyl)-xanthen-9-one
101. 2-methoxy-1,7-bis(3-methylbut-2-enyl)-3,6,8-tris(oxidanyl)xanthen-9-one
102. (c) Paragraph Sign-mangostin Pound>>alpha-mangostin; Nsc 139154; Nsc 27593; Nsc 30552
103. 1,3,6-trihydroxy-7-methoxy-2,8-bis-(3-methyl-2-buten-1-yl)-9h-xanthen-9-one
104. 9h-xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)-
| Molecular Weight | 410.5 g/mol |
|---|---|
| Molecular Formula | C24H26O6 |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 410.17293854 g/mol |
| Monoisotopic Mass | 410.17293854 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 677 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
*Xanthones; Protein Kinase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
/EXPERIMENTAL THERAPY/The mangosteen fruit has a long history of medicinal use in Chinese and Ayurvedic medicine. Recently, the compound a-mangostin, which is isolated from the pericarp of the fruit, was shown to induce cell death in various types of cancer cells in in vitro studies. This led us to investigate the antitumor growth and antimetastatic activities of a-mangostin in an immunocompetent xenograft model of mouse metastatic mammary cancer having a p53 mutation that induces a metastatic spectrum similar to that seen in human breast cancers. Mammary tumors, induced by inoculation of BALB/c mice syngeneic with metastatic BJMC3879luc2 cells, were subsequently treated with a-mangostin at 0, 10 and 20 mg/kg/day using mini-osmotic pumps and histopathologically examined. To investigate the mechanisms of antitumor ability by a-mangostin, in vitro studies were also conducted. Not only were in vivo survival rates significantly higher in the 20 mg/kg/day a-mangostin group versus controls, but both tumor volume and the multiplicity of lymph node metastases were significantly suppressed. Apoptotic levels were significantly increased in the mammary tumors of mice receiving 20 mg/kg/day and were associated with increased expression of active caspase-3 and -9. Other significant effects noted at this dose level were decreased microvessel density and lower numbers of dilated lymphatic vessels containing intraluminal tumor cells in mammary carcinoma tissues. In vitro, a-mangostin induced mitochondria-mediated apoptosis and G1-phase arrest and S-phase suppression in the cell cycle. Since activation by Akt phosphorylation plays a central role in a variety of oncogenic processes, including cell proliferation, anti-apoptotic cell death, angiogenesis and metastasis, we also investigated alterations in Akt phosphorylation induced by a-mangostin treatment both in vitro and in vivo. Quantitative analysis and immunohistochemistry showed that a-mangostin significantly decreased the levels of phospho-Akt-threonine 308 (Thr308), but not serine 473 (Ser473), in both mammary carcinoma cell cultures and mammary carcinoma tissues in vivo. Since lymph node involvement is the most important prognostic factor in breast cancer patients, the antimetastatic activity of a-mangostin as detected in mammary cancers carrying a p53 mutation in the present study may have specific clinical applications. In addition, a-mangostin may have chemopreventive benefits and/or prove useful as an adjuvant therapy, or as a complementary alternative medicine in the treatment of breast cancer.
Shibata M-A et al; BMC Medicine 9: 69 (2011) https://www.biomedcentral.com/1741-7015/9/69
/EXPERIMENTAL THERAPY/This study was conducted to examine the activity of alpha-mangostin against Candida albicans, the most important microorganism implicated in oral candidiasis. Its activity was compared to Clotrimazole and Nystatin. Results showed that alpha-mangostin was effective against C. albicans, the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were 1,000 and 2,000 ug/mL, respectively. The C. albicans killing activity of alpha-mangostin was more effective than Clotrimazole and Nystatin. The cytotoxicity of alpha-mangostin was determined and it was found that alpha-mangostin at 4,000 ug/mL was not toxic to human gingival fibroblast for 480 min. The strong antifungal activity and low toxicity of alpha-mangostin make it a promising agent for treatment of oral candidiasis.
PMID:19776506 Kaomongkolgit R et al; J Oral Sci. 51(3):401-6 (2009)
/EXPERIMENTAL THERAPY/ Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by the accumulation of beta-sheet-rich amyloid oligomers or fibrils which are associated with cellular toxicity in the brain. Inhibition of Abeta aggregation could be a viable therapeutic strategy for slowing and/or preventing the progress of AD. Here /the authors/ reported that a-mangostin (a-M), a polyphenolic xanthone derivative from mangosteen, concentration-dependently attenuated the neurotoxicity induced by Abeta-(1-40) or Abeta-(1-42) oligomers (EC(50) = 3.89 nM, 4.14 nM respectively) as observed by decreased cell viability and impaired neurite outgrowth in primary rat cerebral cortical neurons. Molecular docking and dynamics simulations demonstrated that a-M could potentially bind to Abeta and stabilize alpha-helical conformation. a-M was found to directly dissociate Abeta-(1-40) and Abeta-(1-42) oligomers by blotting with oligomer-specific antibodies. ThioflavinT fluorescence assay and electron microscopy imaging further demonstrated that a-M blocked the fibril formation as well as disturbed the pre-formed fibrils. Taken together, /these/ results indicate that a-M is capable /of/ inhibiting and dissociating the Abeta aggregation, which could contribute to its effect of attenuating Abeta oligomers-induced neurotoxicity. Thus, a-M could be a great potential candidate for AD treatment...
PMID:21958557 Wang Y et al; Neuropharmacology. 62 (2): 871-81 (2012)
ABOUT THIS PAGE
45
PharmaCompass offers a list of Mangostin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mangostin manufacturer or Mangostin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mangostin manufacturer or Mangostin supplier.
PharmaCompass also assists you with knowing the Mangostin API Price utilized in the formulation of products. Mangostin API Price is not always fixed or binding as the Mangostin Price is obtained through a variety of data sources. The Mangostin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mangostin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mangostin, including repackagers and relabelers. The FDA regulates Mangostin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mangostin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mangostin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mangostin supplier is an individual or a company that provides Mangostin active pharmaceutical ingredient (API) or Mangostin finished formulations upon request. The Mangostin suppliers may include Mangostin API manufacturers, exporters, distributors and traders.
click here to find a list of Mangostin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Mangostin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mangostin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mangostin GMP manufacturer or Mangostin GMP API supplier for your needs.
A Mangostin CoA (Certificate of Analysis) is a formal document that attests to Mangostin's compliance with Mangostin specifications and serves as a tool for batch-level quality control.
Mangostin CoA mostly includes findings from lab analyses of a specific batch. For each Mangostin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mangostin may be tested according to a variety of international standards, such as European Pharmacopoeia (Mangostin EP), Mangostin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mangostin USP).
