
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
0
API
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
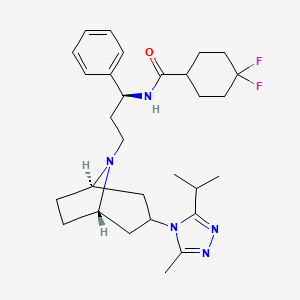

1. 4,4-difluoro-n-((1s)-3-(exo-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)oct-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
2. Selzentry
3. Uk 427,857
4. Uk 427857
5. Uk-427,857
6. Uk-427857
7. Uk427,857
8. Uk427857
1. 376348-65-1
2. Selzentry
3. Celsentri
4. Uk-427857
5. Uk-427,857
6. Uk 427857
7. Md6p741w8a
8. Chembl256907
9. 4,4-difluoro-n-[(1s)-3-[(1r,5s)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide
10. Mvc
11. Chembl1201187
12. Chebi:63608
13. Ncgc00183109-02
14. 4,4-difluoro-n-((1s)-3-((1r,5s)-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexane-1-carboxamide
15. Celsentri(tm)
16. Selzentry(tm)
17. [3h]maraviroc
18. 4,4-difluoro-n-((1s)-3-((1r,5s)-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
19. 4,4-difluoro-n-[(1s)-3-{(3-exo)-3-[3-methyl-5-(propan-2-yl)-4h-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl}-1-phenylpropyl]cyclohexanecarboxamide
20. Pro 140 & Maraviroc
21. Maravirocum
22. Maraviroc [inn:ban:jan]
23. Unii-md6p741w8a
24. Rel-maraviroc
25. Hsdb 8021
26. 4,4-difluoro-n-[(1s)-3-[(1s,5r)-3-(3-isopropyl-5-methyl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenyl-propyl]cyclohexanecarboxamide
27. Uk-427,857 Maraviroc (mvc)
28. Maraviroc [inn]
29. Maraviroc [jan]
30. Maraviroc [mi]
31. [3h]uk 427,857
32. [3h]uk-427,857
33. Maraviroc [vandf]
34. Maraviroc [mart.]
35. (non-labelled)maraviroc-d6
36. Maraviroc [who-dd]
37. Dsstox_cid_28875
38. Dsstox_rid_83144
39. Dsstox_gsid_48949
40. Schembl51991
41. Gtpl803
42. Gtpl806
43. Maraviroc [ema Epar]
44. Cyclohexanecarboxamide, 4,4-difluoro-n-((1s)-3-((3-exo)-3-(3-methyl-5-(1-methylethyl)-4h-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)oct-8-yl)-1-phenylpropyl)-
45. Isopropyl, 4,4-difluoro-n-((1s)-3-{(1r,3s,5s)-3-(3-methyl-5-(propan-2-yl)-4h-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)octan-8-yl}-1-phenylpropyl)cyclohexanecarboxamide
46. Mls006011960
47. Chembl584744
48. Schembl2177194
49. Schembl4576508
50. Maraviroc [orange Book]
51. Dtxsid8048949
52. Maraviroc, >=98% (hplc)
53. Ex-a200
54. Chebi:184662
55. Amy12578
56. Zinc3817234
57. Tox21_113369
58. Ac-558
59. Bdbm50334986
60. Bdbm50464147
61. Akos025402143
62. Akos032960315
63. Zinc100003902
64. Zinc101160855
65. Cs-0366
66. Db04835
67. Ncgc00183109-01
68. As-75265
69. Exo-4,4-difluoro-n-[3-[3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(s)-phenylpropyl]cyclohexanecarboxamide
70. Hy-13004
71. Pro 140 (anti-ccr5 Monoclonal Antibody) & Exo-4,4-difluoro-n-[3-[3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(s)-phenylpropyl]cyclohexanecarboxamide
72. Smr004703532
73. Cas-376348-65-1
74. 348m651
75. Q421369
76. Sr-01000942244
77. J-521678
78. Sr-01000942244-1
79. Brd-a23284911-001-02-4
80. Z1618161028
81. 4,4-difluoro-cyclohexanecarboxylic Acid {(s)-3-[(1s,5s)-3-(3-isopropyl-5-methyl-[1,2,4]triazol-4-yl)-8-aza-bicyclo[3.2.1]oct-8-yl]-1-phenyl-propyl}-amide
82. 4,4-difluoro-n-((1s)-3-((1r,3s,5s)-3-(3-methyl-5-(propan-2-yl)-4h-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
83. 4,4-difluoro-n-((1s)-3-(3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
84. 4,4-difluoro-n-((s)-3-((1r,3r,5s)-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexane-1-carboxamide
85. 4,4-difluoro-n-((s)-3-((1s,3r,5r)-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-aza-bicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
86. 4,4-difluoro-n-((s)-3-((1s,3s,5r)-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-aza-bicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
87. 4,4-difluoro-n-((s)-3-(3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-aza-bicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide
88. 4,4-difluoro-n-[(1s)-3-[(1r,3s,5s)-3-[3-methyl-5-(propan-2-yl)-4h-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide
89. 4,4-difluoro-n-[(1s)-3-[(1r,5s)-3-(3-isopropyl-5-methyl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenyl-propyl]cyclohexanecarboxamide
90. 4,4-difluoro-n-[(1s)-3-[(1r,5s)-3-[3-methyl-5-(propan-2-yl)-4h-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide
91. 4,4-difluoro-n-[(1s)-3-[(1r,5s)-3-[3-methyl-5-(propan-2-yl)-4h-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboximidic Acid
92. 4,4-difluoro-n-{(1s)-3-[(3-exo)-3-(3-isopropyl-5-methyl-4h-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl}cyclohexanecarboxamide
93. 4,4-diluoro-n-[(1s)-3-[(1s,5r)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide
94. Cyclohexanecarboxamide,4,4-difluoro-n-[(1s)-3-[(3-exo)-3-[3-methyl-5-(1-methylethyl)-4h-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl]-
95. Mrv
| Molecular Weight | 513.7 g/mol |
|---|---|
| Molecular Formula | C29H41F2N5O |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 513.32791727 g/mol |
| Monoisotopic Mass | 513.32791727 g/mol |
| Topological Polar Surface Area | 63 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 751 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Selzentry |
| PubMed Health | Maraviroc (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | SELZENTRY (maraviroc) is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Blocking this interaction prevents CCR5-tropic HIV-1 entry into cells.SELZENTRY is available as film-coated tabl... |
| Active Ingredient | Maraviroc |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg; 300mg |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Selzentry |
| PubMed Health | Maraviroc (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | SELZENTRY (maraviroc) is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Blocking this interaction prevents CCR5-tropic HIV-1 entry into cells.SELZENTRY is available as film-coated tabl... |
| Active Ingredient | Maraviroc |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg; 300mg |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
Receptors, CCR5/antagonists & inhibitors; HIV Fusion Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Maraviroc, in combination with other antiretroviral agents, is indicated for adult patients infected with only CCR5-tropic HIV-1. This indication is based on analyses of plasma HIV-1 RNA levels in two controlled studies of maraviroc in treatment-experienced subjects and one study in treatment-naive subjects. Both studies in treatment-experienced subjects were conducted in clinically advanced, 3-class antiretroviral-experienced (NRTI, NNRTI, PI, or enfuvirtide) adults with evidence of HIV-1 replication despite ongoing antiretroviral therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
/BOXED WARNING/ Hepatotoxicity has been reported with maraviroc use. Evidence of a systemic allergic reaction (e.g., pruritic rash, eosinophilia or elevated IgE) prior to the development of hepatotoxicity may occur. Patients with signs or symptoms of hepatitis or allergic reaction following use of maraviroc should be evaluated immediately.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Adult patients infected with only CCR5-tropic HIV-1 should use Maraviroc.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Tropism testing must be conducted with a highly sensitive tropism assay that has demonstrated the ability to identify patients appropriate for Maraviroc use. Outgrowth of pre-existing low-level CXCR4- or dual/mixed-tropic HIV-1 not detected by tropism testing at screening has been associated with virologic failure on Maraviroc.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Use of Maraviroc is not recommended in subjects with dual/mixed or CXCR4-tropic HIV-1 as efficacy was not demonstrated in a phase 2 study of this patient group.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
For more Drug Warnings (Complete) data for Maraviroc (26 total), please visit the HSDB record page.
For treatment-experienced adult patients infected with only CCR5-tropic HIV-1 detectable, who have evidence of viral replication and HIV-1 strains resistant to multiple antiretroviral agents.
FDA Label
Celsentri, in combination with other antiretroviral medicinal products, is indicated for treatment experienced adults, adolescents and children of 2 years of age and older and weighing at least 10 kg infected with only CCR5-tropic HIV-1 detectable
Maraviroc is a chemokine receptor antagonist drug developed by the drug company Pfizer that is designed to act against HIV by interfering with the interaction between HIV and CCR5.
CCR5 Receptor Antagonists
Compounds and drugs that inhibit or block the activity of CCR5 RECEPTORS. (See all compounds classified as CCR5 Receptor Antagonists.)
HIV Fusion Inhibitors
Inhibitors of the fusion of HIV to host cells, preventing viral entry. This includes compounds that block attachment of HIV ENVELOPE PROTEIN GP120 to CD4 RECEPTORS. (See all compounds classified as HIV Fusion Inhibitors.)
J05AX09
J05AX09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX09 - Maraviroc
Absorption
The absolute oral bioavailability of a 100 mg dose is 23% and is predicted to be 33% at 300 mg. Coadministration of a 300mg tablet with a high fat breakfast reduced maraviroc Cmax and AUC by 33% in healthy volunteers.
Volume of Distribution
194 L
... Maraviroc (Celsentri) is an orally administered drug ... Maraviroc plasma exposure is not dose proportional. After a rapid (but moderate) intestinal absorption, several inactive oxidized metabolites are produced via cytochrome P450 3A4 pathway. ...
PMID:18455057 Peytavin G; Med Mal Infect 38 Suppl 1: S12-6 (2008)
Peak maraviroc plasma concentrations are attained 0.5-4 hr following single oral doses of 1-1200 mg administered to uninfected volunteers.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
The absolute bioavailability of a 100 mg dose is 23% and is predicted to be 33% at 300 mg. Maraviroc is a substrate for the efflux transporter P-glycoprotein.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Coadministration of a 300mg tablet with a high fat breakfast reduced maraviroc Cmax and AUC by 33% in healthy volunteers. There were no food restrictions in the studies that demonstrated the efficacy and safety of maraviroc. Therefore, maraviroc can be taken with or without food at the recommended dose.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
For more Absorption, Distribution and Excretion (Complete) data for Maraviroc (12 total), please visit the HSDB record page.
In vitro studies indicate that CYP3A is the major enzyme responsible for maraviroc metabolism.
Maraviroc is the major circulating component ( approximately 42% drug-related radioactivity) following a single oral dose of 300 mg(14)C-maraviroc. The most significant circulating metabolite in humans is a secondary amine (approximately 22% radioactivity) formed by N-dealkylation. This polar metabolite has no significant pharmacological activity. Other metabolites are products of mono-oxidation and are only minor components of plasma drug-related radioactivity.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Studies in humans and in vitro studies using human liver microsomes and expressed enzymes have demonstrated that maraviroc is principally metabolized by the cytochrome P450 system to metabolites that are essentially inactive against HIV-1. In vitro studies indicate that CYP3A is the major enzyme responsible for maraviroc metabolism. In vitro studies also indicate that polymorphic enzymes CYP2C9, CYP2D6 and CYP2C19 do not contribute significantly to the metabolism of maraviroc.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
... Maraviroc is extensively metabolized by CYP3A4, with renal clearance accounting for approximately 23% of total clearance. ...
PMID:19704163 Abel S et al; Antivir Ther 14 (5): 607-18 (2009)
14-18 hours
The terminal half-life of maraviroc following oral dosing to steady state in healthy subjects was 14-18 hours.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
... The half-life of maraviroc is approximately 16 hr. ...
PMID:19704163 Abel S et al; Antivir Ther 14 (5): 607-18 (2009)
Maraviroc is an entry inhibitor and works by blocking HIV from entering human cells. Specifically maraviroc is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Maraviroc selectively binds to the human chemokine receptor CCR5 present on the membrane of CD4 cells (T-cells), preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells.
Maraviroc, a synthetic antiretroviral agent, is an HIV entry inhibitor. The drug is a small molecule CCR5 antagonist. HIV enters host cells by attaching to the CD4+ T-cell receptor using 1 of 2 chemokine co-receptors, CCR5 or CXCR4. Maraviroc selectively binds to CCR5 on the cell membrane and prevents the interaction of HIV-1 glycoprotein 120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells. Maraviroc does not inhibit entry of CXCR4-tropic and dual/mixed-tropic HIV-1 into cells. CCR5 is a co-receptor for the most commonly transmitted HIV-1 strains that predominate during the early stages of infection; this form remains the dominant form in many patients with late-stage infection. Maraviroc is active against some strains of HIV-1 resistant to nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), HIV protease inhibitors (PIs), and HIV entry and fusion inhibitors (enfuvirtide). HIV-1 strains with reduced susceptibility to maraviroc have been produced in vitro and have emerged during maraviroc therapy.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 654
Maraviroc is a member of a therapeutic class called CCR5 co-receptor antagonists. Maraviroc selectively binds to the human chemokine receptor CCR5 present on the cell membrane, preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells. CXCR4-tropic and dual-tropic HIV-1 entry is not inhibited by maraviroc.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Maraviroc inhibits the replication of CCR5-tropic laboratory strains and primary isolates of HIV-1 in models of acute peripheral blood leukocyte infection. The mean EC50 value (50% effective concentration) for maraviroc against HIV-1 group M isolates (subtypes A to J and circulating recombinant form AE) and group O isolates ranged from 0.1 to 4.5 nM (0.05 to 2.3 ng/mL) in cell culture. ... Maraviroc was not active against CXCR4-tropic and dual-tropic viruses (EC50 value >10 uM). The antiviral activity of maraviroc against HIV-2 has not been evaluated.
US Natl Inst Health; DailyMed. Current Medication Information for SELZENTRY (maraviroc) tablet, film coated (May 2010). Available from, as of February 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a94a9a2b-337b-4c13-8622-fc392194dc21
Maraviroc (MVC, Celsentri) is an allosteric and reversible inhibitor of the CCR5 chemokine coreceptor. ... MVC exclusively inhibits the replication of R5- tropic HIV-1 variants after binding to the transmembrane CCR5 receptor cavity.
PMID:19133216 Soriano V, Poveda E; Enferm Infecc Microbiol Clin 26 Suppl 11: 12-6 (2008)
For more Mechanism of Action (Complete) data for Maraviroc (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
41
PharmaCompass offers a list of Maraviroc API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Maraviroc manufacturer or Maraviroc supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Maraviroc manufacturer or Maraviroc supplier.
PharmaCompass also assists you with knowing the Maraviroc API Price utilized in the formulation of products. Maraviroc API Price is not always fixed or binding as the Maraviroc Price is obtained through a variety of data sources. The Maraviroc Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Maraviroc manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Maraviroc, including repackagers and relabelers. The FDA regulates Maraviroc manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Maraviroc API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Maraviroc manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Maraviroc supplier is an individual or a company that provides Maraviroc active pharmaceutical ingredient (API) or Maraviroc finished formulations upon request. The Maraviroc suppliers may include Maraviroc API manufacturers, exporters, distributors and traders.
click here to find a list of Maraviroc suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Maraviroc DMF (Drug Master File) is a document detailing the whole manufacturing process of Maraviroc active pharmaceutical ingredient (API) in detail. Different forms of Maraviroc DMFs exist exist since differing nations have different regulations, such as Maraviroc USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Maraviroc DMF submitted to regulatory agencies in the US is known as a USDMF. Maraviroc USDMF includes data on Maraviroc's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Maraviroc USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Maraviroc suppliers with USDMF on PharmaCompass.
A Maraviroc written confirmation (Maraviroc WC) is an official document issued by a regulatory agency to a Maraviroc manufacturer, verifying that the manufacturing facility of a Maraviroc active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Maraviroc APIs or Maraviroc finished pharmaceutical products to another nation, regulatory agencies frequently require a Maraviroc WC (written confirmation) as part of the regulatory process.
click here to find a list of Maraviroc suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Maraviroc as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Maraviroc API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Maraviroc as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Maraviroc and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Maraviroc NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Maraviroc suppliers with NDC on PharmaCompass.
Maraviroc Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Maraviroc GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Maraviroc GMP manufacturer or Maraviroc GMP API supplier for your needs.
A Maraviroc CoA (Certificate of Analysis) is a formal document that attests to Maraviroc's compliance with Maraviroc specifications and serves as a tool for batch-level quality control.
Maraviroc CoA mostly includes findings from lab analyses of a specific batch. For each Maraviroc CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Maraviroc may be tested according to a variety of international standards, such as European Pharmacopoeia (Maraviroc EP), Maraviroc JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Maraviroc USP).
