

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
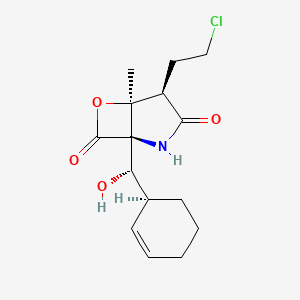

1. Homosalinosporamide A
2. Npi 0052
3. Npi-0052
4. Salinosporamide A
1. Salinosporamide A
2. 437742-34-2
3. Npi-0052
4. (-)-salinosporamide A
5. Npi 0052
6. Ml 858
7. Salinosporamide A (npi-0052, Marizomib)
8. 703p9ydp7f
9. Chebi:48045
10. (1r,4r,5s)-4-(2-chloroethyl)-1-((s)-((s)-cyclohex-2-en-1-yl)(hydroxy)methyl)-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione
11. (1r,4r,5s)-4-(2-chloroethyl)-1-[(s)-(1s)-cyclohex-2-en-1-yl(hydroxy)methyl]-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione
12. Marizomib [usan:inn]
13. Marizomibum
14. Unii-703p9ydp7f
15. Homosalinosporamide A
16. Marizomib [inn]
17. Marizomib (usan/inn)
18. Marizomib [usan]
19. Marizomib [who-dd]
20. Schembl151667
21. Salinosporamide A [mi]
22. Chembl371405
23. Dtxsid00904019
24. (1s,2r,5r)-2-(2-chloroethyl)-5-[(s)-[(1s)-cyclohex-2-en-1-yl]-hydroxymethyl]-1-methyl-7-oxa-4-azabicyclo[3.2.0]heptane-3,6-dione
25. Ex-a3259
26. Zinc3990364
27. Bdbm50398608
28. Akos027323566
29. Db11762
30. Hy-10985
31. Cs-0002986
32. D09640
33. A872651
34. Q7404722
35. (1r,4r,5s)-4-(2-chloroethyl)-1-((s)-((1s)-cyclohex-2-en-1-yl)hydroxymethyl)-5-methyl- 6-oxa-2-azabicyclo(3.2.0)heptane-3,7-dione
36. (1r,4r,5s)-4-(2-chloroethyl)-1-((s)-((s)-cyclohex-2-enyl)(hydroxy)methyl)-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione
37. (1r,4r,5s)-4-(2-chloroethyl)-1-[(s)-[(1s)-cyclohex-2-en-1-yl]-hydroxymethyl]-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione
38. 6-oxa-2-azabicyclo(3.2.0)heptane-3,7-dione, 4-(2-chloroethyl)-1-((s)-(1s)-2-cyclohexen-1-ylhydroxymethyl)-5-methyl-, (1r,4r,5s)-
39. 6-oxa-2-azabicyclo(3.2.0)heptane-3,7-dione, 4-(2-chloroethyl)-1-((s)-(1s)-2-cyclohexen-1-ylhydroxymethyl)-5-methyl-, (1r,4r,5s)-
| Molecular Weight | 313.77 g/mol |
|---|---|
| Molecular Formula | C15H20ClNO4 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 313.1080858 g/mol |
| Monoisotopic Mass | 313.1080858 g/mol |
| Topological Polar Surface Area | 75.6 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 508 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of malignant glial tumours
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
