
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
Regulatory FDF Prices
NA
0
API
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
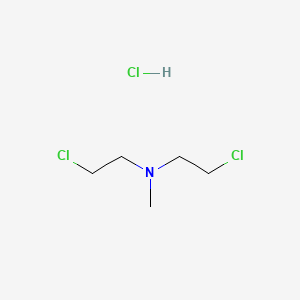

1. Bis(2-chloroethyl)methylamine
2. Caryolysine
3. Chlorethazine
4. Chlormethine
5. Cloramin
6. Embichin
7. Hydrochloride N-oxide, Mechlorethamine
8. Hydrochloride, Mechlorethamine
9. Mechlorethamine
10. Mechlorethamine Hydrochloride N Oxide
11. Mechlorethamine Hydrochloride N-oxide
12. Mechlorethamine N Oxide
13. Mechlorethamine N-oxide
14. Mechlorethamine Oxide
15. Methylchlorethamine
16. Mitomen
17. Mustargen
18. Mustine
19. N-oxide, Mechlorethamine Hydrochloride
20. N-oxide, Nitrogen Mustard
21. Nitrogen Mustard
22. Nitrogen Mustard N Oxide
23. Nitrogen Mustard N-oxide
24. Nitrogranulogen
25. Nitromin
26. Nsc 10107
27. Nsc 762
28. Nsc-10107
29. Nsc-762
30. Nsc10107
31. Nsc762
1. 55-86-7
2. Chlormethine Hydrochloride
3. Mechlorethamine Hcl
4. Nitrogen Mustard Hydrochloride
5. Chlorethamine
6. 2-chloro-n-(2-chloroethyl)-n-methylethanamine Hydrochloride
7. Hn2 Hydrochloride
8. Chlormethinum
9. Stickstofflost
10. Chloramin
11. Mustine Hydrochloride
12. Bis(2-chloroethyl)methylamine Hydrochloride
13. Azotoyperite
14. Dichloren
15. Embikhine
16. Mitoxine
17. Dimitan
18. Erasol
19. Nitol
20. Dema
21. Nitol Takeda
22. Erasol-ido
23. Chlormethine Hcl
24. Caryolysine
25. Mustin Hydrochloride
26. Embichin
27. Nitrogranulogen Hydrochloride
28. N-mustard
29. Mechlorethamine Chloridrate
30. N-methylbis(2-chloroethyl)amine Hydrochloride
31. Stickstofflost-ebewe
32. 2,2'-dichloro-n-methyldiethylamine Hydrochloride
33. Dichloromethyldiethylamine Hydrochloride
34. 1,5-dichloro-3-methyl-3-azapentane Hydrochloride
35. Di(2-chloroethyl)methylamine Hydrochloride
36. Methyldi(2-chloroethyl)amine Hydrochloride
37. Nci-c56382
38. Mba Hydrochloride
39. Methylbis(2-chloroethyl)amine Hydrochloride
40. Ethanamine, 2-chloro-n-(2-chloroethyl)-n-methyl-, Hydrochloride
41. Chlormethine (hydrochloride)
42. Sk 101
43. N-methyl-2,2'-dichlorodiethylamine Hydrochloride
44. Nsc-762
45. Methyldi(beta-chloroethyl)amine Hydrochloride
46. N-methyl-di-2-chloroethylamine Hydrochloride
47. N-lost
48. Methyl-bis(beta-chloroethyl)amine Hydrochloride
49. N-methyl-bis-beta-chlorethylamine Hydrochloride
50. C 6866
51. N,n-bis(2-chloroethyl)methylamine Hydrochloride
52. Nitrogen Mustard (hn-2), Hydrochloride
53. L0mr697hhi
54. Beta,beta'-dichlorodiethyl-n-methylamine Hydrochloride
55. Nsc762
56. 2-chloro-n-(2-chloroethyl)-n-methylethanamine Hydrochlroide
57. Chebi:55368
58. N,n-bis(2-chloraethyl)methylamin-hydrochlorid
59. Bis(2-chloroethyl)-n-methylamine Hydrochloride
60. Mechlorethamine Hydrochloride [usp]
61. Carolysine
62. Mebichloramine
63. Nitrogranulogen
64. Embechine
65. Valchlor
66. Ncgc00091835-01
67. 2-chloro-n-(2-chloroethyl)-n-methylethanamine;hydrochloride
68. Embiquine
69. Zagreb
70. Pliva
71. Bis-(2-chloroethyl)methylamine Hydrochloride
72. Dsstox_cid_5757
73. Dsstox_rid_77910
74. Dsstox_gsid_25757
75. Kloramin (van)
76. N-mustard [german]
77. Mechlorethamine Hydrochloride (usp)
78. Cas-55-86-7
79. Ccris 448
80. Dichloren Hydrochloride
81. Hsdb 7176
82. Einecs 200-246-0
83. Unii-l0mr697hhi
84. Ai3-16195
85. Mustine Hydrochlor
86. Nitol 'takeda'
87. Mustargen (tn)
88. Prestwick_37
89. Valchlor (tn)
90. Erasol Hydrochloride
91. Mfcd00012517
92. Nitrogen Mustard Hcl
93. Embichin Hydrochloride
94. Chloramin Hydrochloride
95. Mustargen Hydrochloride
96. N,n-bis(2-chloraethyl)methylamin-hydrochlorid [german]
97. Caryolysine Hydrochloride
98. Nsc-762 Hydrochloride
99. Chlormethini Hydrochloridum
100. Chloromethine Hydrochloride
101. Mechorethamine Hydrochloride
102. Schembl3855
103. Mls003899210
104. Diethylamine, 2,2'-dichloro-n-methyl, Hydrochloride
105. Chembl1201001
106. Dtxsid8025757
107. Wln: G2n1&2g &gh
108. Mechlorethamine Hydrochloride, 98%
109. Hy-b1253
110. Tox21_111170
111. Tox21_200638
112. S4252
113. Akos015915356
114. Tox21_111170_1
115. Ccg-264667
116. Cs-5080
117. Bis(2-chloroethyl)methylammonium Chloride
118. Ncgc00091835-10
119. Ncgc00258192-01
120. Bp-20221
121. Chlormethine Hydrochloride [mart.]
122. Mechlorethamine Hydrochloride [mi]
123. Smr000059226
124. Chlormethine Hydrochloride [who-dd]
125. Chlormethine Hydrochloride [who-ip]
126. Db-007290
127. Mechlorethamine Hydrochloride [hsdb]
128. Bis(2-chloroethyl)-methylamine Hydrochloride
129. Bis(2-chloroethyl)methyl Amine Hydrochloride
130. Mechlorethamine Hydrochloride [vandf]
131. N,n-bis(chloroethyl)methylamine Hydrochloride
132. Sw220100-1
133. Bis (2-chloroethyl)-methylamine Hydrochloride
134. Bis (2-chloroethyl)methyl Amine Hydrochloride
135. Bis-(2-chloroethyl)-methylamine Hydrochloride
136. Mechlorethamine Hydrochloride [usp-rs]
137. N-methylbis (2-chloroethyl)amine Hydrochloride
138. Bis-(2-chloroethyl)-methyl-amine Hydrochloride
139. D04872
140. F11444
141. Methyldi(.beta.-chloroethyl)amine Hydrochloride
142. N-methyl-bis-(2-chloroethyl)amine Hydrochloride
143. N-methylbis(2-chloroethyl)-amine Hydrochloride
144. 2,2'-dichloro-n-methyldiethylamino Hydrochloride
145. Bis(2-chloroethyl)methylamine-[d4] Hydrochloride
146. Bis-(2-chloro-ethyl)-methyl-amine Hydrochloride
147. Diethylamine,2'-dichloro-n-methyl, Hydrochloride
148. Methylbis(.beta.-chloroethyl)amine Hydrochloride
149. Chlormethini Hydrochloridum [who-ip Latin]
150. Diethylamine,2'-dichloro-n-methyl-, Hydrochloride
151. Mechlorethamine Hydrochloride [orange Book]
152. Methyl Bis(.beta.-chloroethyl)amine, Hydrochloride
153. N-methylbis(.beta.-chlorethyl)amine Hydrochloride
154. 1,5-dichloro-3-methyl-3-azapentane, Hydrochloride
155. Mechlorethamine Hydrochloride [usp Monograph]
156. N,n-bis(2-chloroethyl)-n-methylamine Hydrochloride
157. W-105542
158. Q27124263
159. .beta.,.beta.'-dichlorodiethyl-n-methylamine Hydrochloride
160. 2-chloro-n-(chloroethyl)-n-methylethanamine Hydrochloride
161. 2-chloro-n-(2 -chloroethyl)-n-methylethanamine Hydrochloride
162. 2-chloro-n-(2-chloroethyl) -n-methylethanamine Hydrochloride
163. 2-chloro-n-(2-chloroethyl)-n-methylethanamine Hydrochloride (1:1)
| Molecular Weight | 192.5 g/mol |
|---|---|
| Molecular Formula | C5H12Cl3N |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 4 |
| Exact Mass | 191.003532 g/mol |
| Monoisotopic Mass | 191.003532 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 43.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Alkylating Agents; Antineoplastic Agents, Alkylating;
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antineoplastic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1032
VET: Antineoplastic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1032
MEDICATION (VET): ... MECHLORETHAMINE HYDROCHLORIDE IS REPORTED TO BE USED FOR TREATMENT OF LYMPHOSARCOMA & OF MAST CELL SARCOMA IN DOGS & OF FOWL LEUKOSIS ... /MECHLORETHAMINE HYDROCHLORIDE/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 198 (1975)
For more Therapeutic Uses (Complete) data for MECHLORETHAMINE HYDROCHLORIDE (11 total), please visit the HSDB record page.
Adverse CNS effects which have occurred following IV administration of mechlorethamine include weakness, headache, drowsiness, vertigo, lightheadedness, convulsions, progressive muscle paralysis, paresthesia, cerebral degeneration, coma, and death. Serious neurotoxicity appears to be a problem only when high doses or intra-arterial and regional perfusion administration techniques are used. Immediate and delayed neurotoxicity, sometimes severe, has been reported in patients receiving higher than recommended doses of the drug in preparation for bone marrow transplantation; neurotoxicity appeared to increase with age and dose administered and occurred more frequently in patients who also received procarbazine or cyclophosphamide.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1069
Adverse dermatologic effects of systemic mechlorethamine therapy occasionally include a maculopapular skin eruption which is apparently idiosyncratic. The maculopapular skin eruption does not necessarily recur with subsequent doses and is not a contraindication to future use of the drug. Erythema multiforme also has been reported. Hypersensitivity reactions, including anaphylaxis, have been reported in patients receiving IV mechlorethamine. ... Herpes zoster, which occurs commonly in patients with lymphoma, may be precipitated by treatment with mechlorethamine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1069
Major and dose-limiting adverse effects of mechlorethamine are nausea and vomiting, which occur in up to 90% of patients who receive the drug and are presumably due to CNS stimulation.Vomiting, which may be severe enough to precipitate vascular accidents in patients with hemorrhagic tendencies, occurs within 0.5-8 hours (usually 1-3 hours) after administration of mechlorethamine. Emesis generally subsides within 8 hours, but nausea may persist 24 hours or longer. ... Other GI effects of mechlorethamine include anorexia, diarrhea, severe hematemesis and dehydration secondary to vomiting, and rarely, peptic ulcers.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1069
Mechlorethamine must be used with extreme caution in patients with leukopenia, thrombocytopenia, or anemia caused by infiltration of bone marrow with malignant cells. In these patients, a good response to mechlorethamine therapy with disappearance of tumor from the bone marrow may be associated with improved bone marrow function; however, in the absence of good response or in patients who have received previous treatment with antineoplastic agents, hematopoiesis may be further compromised, resulting in more severe leukopenia, thrombocytopenia, anemia, and possibly death. Patients with chronic lymphocytic leukemia appear to be especially sensitive to the hematopoietic effects of mechlorethamine and should receive the drug with extreme caution, if at all.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1069
For more Drug Warnings (Complete) data for MECHLORETHAMINE HYDROCHLORIDE (17 total), please visit the HSDB record page.
Alkylating Agents
Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning. Many are used as antineoplastic agents, but most are very toxic, with carcinogenic, mutagenic, teratogenic, and immunosuppressant actions. They have also been used as components in poison gases. (See all compounds classified as Alkylating Agents.)
Irritants
Drugs that act locally on cutaneous or mucosal surfaces to produce inflammation; those that cause redness due to hyperemia are rubefacients; those that raise blisters are vesicants and those that penetrate sebaceous glands and cause abscesses are pustulants; tear gases and mustard gases are also irritants. (See all compounds classified as Irritants.)
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
Chemical Warfare Agents
Chemicals that are used to cause the disturbance, disease, or death of humans during WARFARE. (See all compounds classified as Chemical Warfare Agents.)
Following IV injection, the drug undergoes rapid chemical transformation and unchanged mechlorethamine is undetectable in the blood within a few minutes. Less than 0.01% of an IV dose is excreted unchanged in the urine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1070
Mice given 35 mg/kg body wt mechlorethamine hydrochloride iv and examined by autoradiography had significant levels of compound in brain, spinal cord, lung and submaxillary glands.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 201 (1975)
Mechlorethamine is incompletely absorbed following intracavitary administration, probably because of rapid deactivation by body fluids.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1804
Following its in vivo admin, mechlorethamine or its hydrochloride is probably converted into ethyleneimmonium ion which reacts with guanine residues in /either the same or/ adjacent strands of DNA as well as with SH groups.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 201 (1975)
Mechlorethamine, as an alkylating agent, interferes with DNA replication and transcription of RNA and ultimately results in the disruption of nucleic acid function. Mechlorethamine also possesses weak immunosuppressive activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1070
Mechlorethamine, as an alkylating agent, interferes with DNA replication and transcription of RNA, and ultimately results in the disruption of nucleic acid function.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 694
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
44
PharmaCompass offers a list of Mechlorethamine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mechlorethamine manufacturer or Mechlorethamine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mechlorethamine manufacturer or Mechlorethamine supplier.
PharmaCompass also assists you with knowing the Mechlorethamine API Price utilized in the formulation of products. Mechlorethamine API Price is not always fixed or binding as the Mechlorethamine Price is obtained through a variety of data sources. The Mechlorethamine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mechlorethamine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mechlorethamine, including repackagers and relabelers. The FDA regulates Mechlorethamine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mechlorethamine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mechlorethamine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mechlorethamine supplier is an individual or a company that provides Mechlorethamine active pharmaceutical ingredient (API) or Mechlorethamine finished formulations upon request. The Mechlorethamine suppliers may include Mechlorethamine API manufacturers, exporters, distributors and traders.
click here to find a list of Mechlorethamine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mechlorethamine DMF (Drug Master File) is a document detailing the whole manufacturing process of Mechlorethamine active pharmaceutical ingredient (API) in detail. Different forms of Mechlorethamine DMFs exist exist since differing nations have different regulations, such as Mechlorethamine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mechlorethamine DMF submitted to regulatory agencies in the US is known as a USDMF. Mechlorethamine USDMF includes data on Mechlorethamine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mechlorethamine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mechlorethamine suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Mechlorethamine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Mechlorethamine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Mechlorethamine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Mechlorethamine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Mechlorethamine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Mechlorethamine suppliers with NDC on PharmaCompass.
Mechlorethamine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mechlorethamine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mechlorethamine GMP manufacturer or Mechlorethamine GMP API supplier for your needs.
A Mechlorethamine CoA (Certificate of Analysis) is a formal document that attests to Mechlorethamine's compliance with Mechlorethamine specifications and serves as a tool for batch-level quality control.
Mechlorethamine CoA mostly includes findings from lab analyses of a specific batch. For each Mechlorethamine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mechlorethamine may be tested according to a variety of international standards, such as European Pharmacopoeia (Mechlorethamine EP), Mechlorethamine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mechlorethamine USP).

