
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
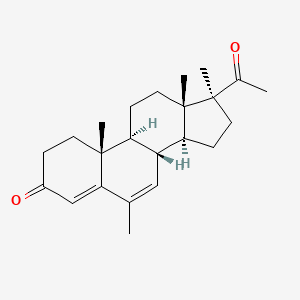

1. Colpro
2. Colprone
3. Prothil
1. Medrogesterone
2. Colprone
3. Prothil
4. Etogyn
5. Colpro
6. Metrogestone
7. 977-79-7
8. 6,17-dimethylpregna-4,6-diene-3,20-dione
9. Ay-62022
10. 6,17-dimethyl-6-dehydroprogesterone
11. Pregna-4,6-diene-3,20-dione, 6,17-dimethyl-
12. Nsc-123018
13. Ay 62022
14. 6-methyl-6-dehydro-17-methylprogesterone
15. 077dn93g5b
16. Ay-13615s
17. Ay-13615
18. Ay 13615s
19. Medrogestona
20. Medrogestonum
21. (8r,9s,10r,13s,14s,17s)-17-acetyl-6,10,13,17-tetramethyl-2,8,9,11,12,14,15,16-octahydro-1h-cyclopenta[a]phenanthren-3-one
22. Medrogestonum [inn-latin]
23. Medrogestona [inn-spanish]
24. Medrogeston
25. Unii-077dn93g5b
26. Medrogestone [usan:inn:ban]
27. Einecs 213-555-0
28. Nsc 123018
29. Brn 2302887
30. Medrogestone [mi]
31. Medrogestone (usan/inn)
32. Medrogestone [inn]
33. Medrogestone [usan]
34. R 13615
35. Medrogestone [mart.]
36. Medrogestone [who-dd]
37. Schembl140614
38. Chembl2106825
39. Dtxsid70878637
40. Chebi:135446
41. Zinc4216820
42. Db09124
43. D04885
44. 977m797
45. Q6807285
46. (8r,9s,10r,13s,14s,17s)-17-acetyl-6,10,13,17-tetramethyl-8,9,10,11,12,13,14,15,16,17-decahydro-1h-cyclopenta[a]phenanthren-3(2h)-one
| Molecular Weight | 340.5 g/mol |
|---|---|
| Molecular Formula | C23H32O2 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 340.240230259 g/mol |
| Monoisotopic Mass | 340.240230259 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 714 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Medrogestone is indicated as adjunct to treat endometial shedding in menopausal women, to treat secondary amenorrhea, to induce menses and to treat dysfunctional uterine bleeding in adult and adolescent women.
Medrogestone was created as a more potent and orally active option of progesterone. In pre-clinical trials, medrogestone was proven to have four times more progestational activity than progesterone with a similar duration effect than the one found for 17-hydroxyprogesterone. Medrogestone was also able to maintain pregnancy and prevented ovulation in ovariectomized rats. Administration of medrogestone, alone or with premarin, prevented pregnancy, as well as it suppressed ovarian weight increase by nearly 100% of the tested individuals. Medrogestone does not produce any androgenic effect but it presented a marked anti-androgenic effect. It did not present an oestrogenic effect, nor changes in organ weight or histological appearance in adrenal glands or thymus and it does not present any anti-inflammatory effects.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03D - Progestogens
G03DB - Pregnadien derivatives
G03DB03 - Medrogestone
Absorption
When administered, medrogestone presents a very rapid gastrointestinal absorption with a bioavailability of 100%. The maximum serum concentration of medrogestone is 10-15 ng/ml.
Route of Elimination
The elimination time of medrogestone is of 36 hours.
The non-protein bound fraction of medrogestoneis available for metabolism. The main route in the metabolism of medrogestone is hydroxylation.
The half-life of medrogestone is reported to be of 4 hours.
Medrogestone is a progestogen, thus its action is done under the same profile. These type of molecules are steroid hormones that bind and activate the progesterone receptor. Its action may involve the suppression of gonadotropic hormones from the anterior portion of the pituitary gland and secondary suppression of testosterone. Medrogestone presents structural similarities to testosterone which allows it to compete for the androgen-receptor-protein receptor sites in prostatic cells. Administration of medrogestone diminishes the response to endogenous hormones in tumor cells due to a reduction in hormone steroid receptors; this effect will translate into cytotoxic or antiproliferative effects.
ABOUT THIS PAGE
98
PharmaCompass offers a list of Medrogestone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Medrogestone manufacturer or Medrogestone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Medrogestone manufacturer or Medrogestone supplier.
PharmaCompass also assists you with knowing the Medrogestone API Price utilized in the formulation of products. Medrogestone API Price is not always fixed or binding as the Medrogestone Price is obtained through a variety of data sources. The Medrogestone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Medrogestone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Medrogestone, including repackagers and relabelers. The FDA regulates Medrogestone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Medrogestone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Medrogestone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Medrogestone supplier is an individual or a company that provides Medrogestone active pharmaceutical ingredient (API) or Medrogestone finished formulations upon request. The Medrogestone suppliers may include Medrogestone API manufacturers, exporters, distributors and traders.
click here to find a list of Medrogestone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Medrogestone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Medrogestone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Medrogestone GMP manufacturer or Medrogestone GMP API supplier for your needs.
A Medrogestone CoA (Certificate of Analysis) is a formal document that attests to Medrogestone's compliance with Medrogestone specifications and serves as a tool for batch-level quality control.
Medrogestone CoA mostly includes findings from lab analyses of a specific batch. For each Medrogestone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Medrogestone may be tested according to a variety of international standards, such as European Pharmacopoeia (Medrogestone EP), Medrogestone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Medrogestone USP).
