
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
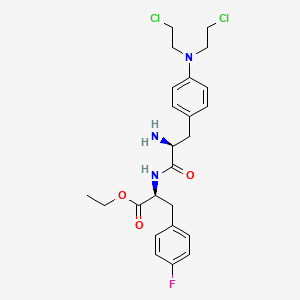

1. Melflufen
2. Melphalan-flufenamide
3. Pepaxto
1. Melflufen
2. Prodrug J 1
3. Prodrug J-1
4. J 1 (prodrug)
5. J-1 (prodrug)
6. 380449-51-4
7. 380449-51-4 (free Base)
8. F70c5k4786
9. J-1
10. L-phenylalanine, 4-(bis(2-chloroethyl)amino)-l-phenylalanyl-4-fluoro-, Ethyl Ester
11. Ethyl (2s)-2-((2s)-2-amino-3-(4-(bis(2-chloroethyl)amino)phenyl)propanamido)-3-(4-fluorophenyl)propanoate
12. J1
13. Mff
14. Ethyl (2s)-2-[[(2s)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoyl]amino]-3-(4-fluorophenyl)propanoate
15. Ethyl (s)-2-((s)-2-amino-3-(4-(bis(2-chloroethyl)amino)phenyl)propanamido)-3-(4-fluorophenyl)propanoate
16. Melphalan Flufenamide [inn]
17. Unii-f70c5k4786
18. Chembl4303060
19. Schembl18239898
20. Gtpl11605
21. Dtxsid40191461
22. Glxc-25713
23. Melphalan Flufenamide (usan/inn)
24. Melphalan Flufenamide [usan:inn]
25. Who 9493
26. Melphalan Flufenamide [usan]
27. Melphalan Flufenamide [who-dd]
28. J 1
29. Hy-105019
30. Cs-0024709
31. D11865
32. Q27277739
| Molecular Weight | 498.4 g/mol |
|---|---|
| Molecular Formula | C24H30Cl2FN3O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 14 |
| Exact Mass | 497.1648254 g/mol |
| Monoisotopic Mass | 497.1648254 g/mol |
| Topological Polar Surface Area | 84.7 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Melphalan flufenamide is indicated in combination with [dexamethasone] to treat adults with relapsed or refractory multiple myeloma who have received 4 therapies and are refractory to at least one proteasome inhibitor, immunomodulatory agent, and anti-CD38 monoclonal antibody. The FDA has withdrawn the drug from the market for this indication following phase 3 trial data showing decreased overall survival.
Pepaxti is indicated, in combination with dexamethasone, for the treatment of adult patients with multiple myeloma who have received at least three prior lines of therapies, whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and one anti-CD38 monoclonal antibody, and who have demonstrated disease progression on or after the last therapy. For patients with a prior autologous stem cell transplantation, the time to progression should be at least 3 years from transplantation (see section 4. 4).
Melphalan flufenamide is an alkylating agent indicated to treat relapsed or refractory multiple myeloma in Melphalan flufenamide has a long duration of action as it is given every 28 days. Patients should be counselled regarding risks of thrombocytopenia, neutropenia, anemia, infections, secondary malignancies, embryo-fetal toxicity.
L01AA10
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AA - Nitrogen mustard analogues
L01AA10 - Melphalan flufenamide
Absorption
For a 40 mg intravenous infusion, the active metabolite reaches a Cmax of 432 ng/mL, with a Tmax of 4-15 minutes, and an AUC of 3143 h\*g/mL.
Route of Elimination
Data regarding the route of elimination of melphalan flufenamide are not readily available. Free melphalan undergoes rapid and spontaneous decomposition, complicating studies on the route of elimination. However, it is expected to be mainly renally excreted.
Volume of Distribution
The mean volume of distribution of melphalan flufenamide is 35 L and of melphalan is 76 L.
Clearance
The mean clearance of melphalan flufenamide is 692 L/h and of melphalan is 23 L/h.
Melphalan flufenamide is metabolised to desethyl-melphalan and melphalan. Melphalan is spontaneously hydrolyzed to monohydroxy-melphalan and dihydroxy-melphalan.
The mean elimination half life of melphalan flufenamide is 2.1 minutes and of melphalan is 70 minutes.
Melphalan flufenamide is a more lipophilic prodrug of melphalan, which allows it to be more readily uptaken by cells. It is likely taken up into malignant cells by passive diffusion, where it is hydrolyzed by aminopeptidase N. The expression of aminopeptidases, along with other hydrolytic enzymes, is upregulated in many malignant cells, making the hydrolysis reaction to melphalan more favourable in a malignant cell. Increased concentrations of free melphalan in malignant cells leads to rapid irreversible DNA damage and apoptosis, reducing the potential for the development of resistance. Free melphalan is an nitrogen mustard derivative alkylating agent. Melphalan attaches alkyl groups to the N-7 position of guanine and N-3 position of adenine, leading to the formation of monoadducts, and DNA fragmenting when repair enzymes attempt to correct the error. It can also cause DNA cross-linking from the N-7 position of one guanine to the N-7 position of another, preventing DNA strands from separating for synthesis or transcription. Finally, melphalan can induce a number of different mutations. While melphalan induces phosphorylation of the DNA damage marker -H2AX in melphalan sensitive cells at 6 hours, melphalan flufenamide induces -H2AX at 2 hours. Melphalan flufenamide is also able to induce -H2AX in melphalan-resistant cells.
ABOUT THIS PAGE
51
PharmaCompass offers a list of Melphalan Flufenamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Melphalan Flufenamide manufacturer or Melphalan Flufenamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Melphalan Flufenamide manufacturer or Melphalan Flufenamide supplier.
PharmaCompass also assists you with knowing the Melphalan Flufenamide API Price utilized in the formulation of products. Melphalan Flufenamide API Price is not always fixed or binding as the Melphalan Flufenamide Price is obtained through a variety of data sources. The Melphalan Flufenamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Melphalan Flufenamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Melphalan Flufenamide, including repackagers and relabelers. The FDA regulates Melphalan Flufenamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Melphalan Flufenamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Melphalan Flufenamide supplier is an individual or a company that provides Melphalan Flufenamide active pharmaceutical ingredient (API) or Melphalan Flufenamide finished formulations upon request. The Melphalan Flufenamide suppliers may include Melphalan Flufenamide API manufacturers, exporters, distributors and traders.
Melphalan Flufenamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Melphalan Flufenamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Melphalan Flufenamide GMP manufacturer or Melphalan Flufenamide GMP API supplier for your needs.
A Melphalan Flufenamide CoA (Certificate of Analysis) is a formal document that attests to Melphalan Flufenamide's compliance with Melphalan Flufenamide specifications and serves as a tool for batch-level quality control.
Melphalan Flufenamide CoA mostly includes findings from lab analyses of a specific batch. For each Melphalan Flufenamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Melphalan Flufenamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Melphalan Flufenamide EP), Melphalan Flufenamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Melphalan Flufenamide USP).
