
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
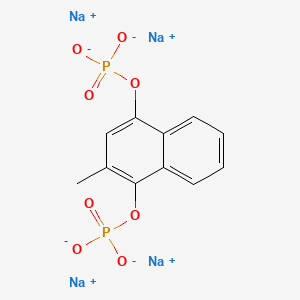

1. 2-methyl-1,4-naphthohydroquinone
2. 2-methyl-1,4-naphthoquinol
3. Dihydrovitamin K3
4. Menadiol
5. Menadiol Diphosphate
6. Menadiol Diphosphate Ion
7. Menadiol Diphosphate, Monosodium Salt
8. Menadiol Diphosphate, Tetrasodium Salt
9. Menadiol, (-1)-ion
10. Menadiol, Monopotassium Salt
11. Naphtadon
12. Reduced Menadione
13. Synkavit
14. Synkavite
1. 131-13-5
2. Kappadione
3. Menadiol Sodium Diphosphate Anhydrous
4. Tetrasodium2-methyl-1,4-naphthylenebis(phosphate)
5. R2l46we615
6. 1,4-naphthalenediol, 2-methyl-, Bis(dihydrogen Phosphate), Tetrasodium Salt
7. Synkavit
8. Kipca Water Soluble
9. Menadiol Sodium Phosphate
10. Menadione Sodium Phosphate
11. Sodium Menadione Diphosphate
12. Menadiol Tetrasodium Diphosphate
13. Einecs 205-012-1
14. Menadione Diphosphate Tetrasodium Salt
15. 1:4-naphthaquinol-bisdisodium Phosphate
16. Unii-r2l46we615
17. Menadiol Sodium Diphosphate Anhydrous [usan]
18. 2-methyl-1,4-naphthaquinol Bis(disodium Phosphate)
19. Tetrasodium 2-methyl-1,4-naphthalenediol Diphosphate
20. Tetrasodium 2-methyl-1,4-naphthohydroquinone Diphosphate
21. 1,4-naphthalenediol, 2-methyl-, Diphosphate, Tetrasodium Salt
22. Tetrasodium 2-methyl-1,4-naphthahydroquinone Diphosphoric Acid Ester
23. Tetrasodium 2-methyl-1,4-naphthalenediol Bis(dihydrogen Phosphate)
24. 2-methyl-1,4-naphthohydroquinone Diphosphoric Acid Ester Tetrasodium Salt
25. Phosphoric Acid, Diester With 2-methyl-1,4-naphthalenediol, Tetrasodium Salt
26. Tetrasodium 2-methyl-1,4-naphthylenebis(phosphate)
27. Dtxsid80156832
28. Db09332
29. 1,4-naphthalenediol, 2-methyl-, Bis(dihydrogen Phosphate), Tetrasodium
30. Menadiol Sodium Diphosphate [mi]
31. Menadiol Sodium Diphosphate [who-dd]
32. Q6367106
33. 1,4-naphthalenediol, 2-methyl-, 1,4-bis(dihydrogen Phosphate), Sodium Salt (1:4)
34. 2-methyl-1,4-naphthalenediol Bis(dihydrogen Phosphate) Tetrasodium Salt
| Molecular Weight | 422.08 g/mol |
|---|---|
| Molecular Formula | C11H8Na4O8P2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 421.92851833 g/mol |
| Monoisotopic Mass | 421.92851833 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 434 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
Anticoagulant-induced prothrombin deficiency caused by coumadin or indanedione derivatives, prophylaxis and therapy of hemorrhagic disease of the newborn, hypoprothrombinemia due to antibacterial therapy, hypoprothrombinemia secondary to factors limiting absorption or synthesis of vitamin K (for example, obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancreas, and regional enteritis, other drug-induced hypoprothrombinemia where it is definitely shown that the result is due to interference with vitamin K metabolism).
Menadiol sodium diphosphate is a highly water-soluble vitamin K analog. The presence of vitamin K is necessary for the formation of prothrombin, factor VII, factor IX and factor X. Lack of vitamin K results in an increased risk of hemorrhage, which can be minor or life-threatening.
Absorption
Menadiol sodium phosphate (vitamin K3), the synthetic analog of vitamin K, being water soluble, is advised in intestinal malabsorption or in states in which bile flow is deficient. The primary disadvantage is that it takes 24 h to initiate therapeutic effects, however, this effect lasts for several days. The dose is 540 mg orally, daily. Menadiol sodium phosphate, even in moderate doses, may lead to hemolytic anemia and, for this reason, neonates should not receive this medication. This precautionary measure is valid especially those that are deficient in glucose 6-phosphate dehydrogenase (G6PD); their immature livers are unable to compensate for the heavy bilirubin load and there is an increased risk of kernicterus.
Route of Elimination
Vitamin K is heavily metabolized in the liver and excreted in the urine and bile. In tracer studies, it was found that approximately 20% of an injected dose of phylloquinone (Vitamin K metabolite) was found in the urine whereas about 40-50 % was excreted in the feces via the biliary system. The proportion of drug excreted was the same regardless of whether the injected dose was 1 mg or 45 g. It can, therefore, be inferred that about 60-70% percent of the amounts of phylloquinone absorbed from each vitamin-K containing meal will be lost to the body by excretion. Two major human excretion products have been identified: carboxylic acids with 5 and 7-carbon sidechains that are excreted in the urine as glucuronide conjugates. The biliary metabolites have not been clearly identified but are initially excreted as water-soluble conjugates and become lipid soluble during their passage through the gut, probably through deconjugation by the gut flora. There is no evidence for body stores of vitamin K being conserved by an enterohepatic circulation. Vitamin K itself is too lipophilic to be excreted in the bile and the sidechain-shortened carboxylic acid metabolites are not biologically active.
Volume of Distribution
In a study of rabbits, the apparent volume of distribution (V(d)/F) in plasma was 30.833 12.835 L.
Clearance
The plasma clearance (CL/F) of VK3 was 0.822 0.254 L min-1. [LI572]
Menadione or 2-methyl-1,4-naphthoquinone is a synthetic vitamin K analog, undergoes 1-electron reduction by enzymes such as microsomal NADPHcytochrome P450 reductase and mitochondrial NADHubiquinone oxidoreductase (complex I), resulting in redox cycling, or it detoxification via two-electron reduction by NAD(P)Hquinone oxidoreductase. Vitamin K is a group of lipophilic, hydrophobic vitamins that exist naturally in two forms (and in 3 synthetic forms): vitamin K1, which is found in plants, and vitamin K2, which is synthesized by bacteria. Vitamin K is an important dietary component because it is necessary as a cofactor in the activation of vitamin K dependent proteins. Metabolism of vitamin K occurs mainly in the liver. In the first step, vitamin K is reduced to its quinone form by a quinone reductase such as NADPH dehydrogenase. Reduced vitamin K is the form required to convert vitamin K dependent protein precursors to their active states. It acts as a cofactor to the integral membrane enzyme vitamin K-dependent gamma-carboxylase (along with water and carbon dioxide as co-substrates), which carboxylates glutamyl residues to gamma-carboxy-glutamic acid residues on certain proteins, activating them. Each converted glutamyl residue produces a molecule of vitamin K epoxide, and certain proteins may have more than one residue requiring carboxylation. To end the cycle, the vitamin K epoxide is returned to vitamin K via the vitamin K epoxide reductase enzyme, also an integral membrane protein. The vitamin K dependent proteins include various important coagulation factors, such as prothrombin. Warfarin and other coumarin drugs act as anticoagulants by blocking vitamin K epoxide reductase.
Mean elimination half-life of menadione was 27.17 min in the plasma of rabbits, in one study.
Menadiol sodium phosphate (vitamin K3) is involved as a cofactor in the posttranslational gamma-carboxylation of glutamic acid residues of various proteins in the body, allowing for propagation of the clotting cascade that results in coagulation. These proteins are comprised of the vitamin K-dependent coagulation factors II (prothrombin), VII (proconvertin), IX (Christmas factor), X (Stuart factor), protein C, protein S, protein Zv and a growth-arrest-specific factor (Gas6). The two vitamin K-dependent proteins found in bone are osteocalcin, also known as bone G1a (gamma-carboxyglutamate) protein or BGP, and the matrix G1a protein or MGP. Gamma-carboxylation is catalyzed by the vitamin K-dependent gamma-carboxylases. The reduced form of vitamin K, vitamin K hydroquinone, is the actual cofactor for the gamma-carboxylases. Proteins containing gamma-carboxyglutamate are called G1a proteins.
Market Place

ABOUT THIS PAGE
72
PharmaCompass offers a list of Menadiol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Menadiol manufacturer or Menadiol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Menadiol manufacturer or Menadiol supplier.
PharmaCompass also assists you with knowing the Menadiol API Price utilized in the formulation of products. Menadiol API Price is not always fixed or binding as the Menadiol Price is obtained through a variety of data sources. The Menadiol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Menadiol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Menadiol, including repackagers and relabelers. The FDA regulates Menadiol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Menadiol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Menadiol supplier is an individual or a company that provides Menadiol active pharmaceutical ingredient (API) or Menadiol finished formulations upon request. The Menadiol suppliers may include Menadiol API manufacturers, exporters, distributors and traders.
click here to find a list of Menadiol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Menadiol DMF (Drug Master File) is a document detailing the whole manufacturing process of Menadiol active pharmaceutical ingredient (API) in detail. Different forms of Menadiol DMFs exist exist since differing nations have different regulations, such as Menadiol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Menadiol DMF submitted to regulatory agencies in the US is known as a USDMF. Menadiol USDMF includes data on Menadiol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Menadiol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Menadiol suppliers with USDMF on PharmaCompass.
Menadiol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Menadiol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Menadiol GMP manufacturer or Menadiol GMP API supplier for your needs.
A Menadiol CoA (Certificate of Analysis) is a formal document that attests to Menadiol's compliance with Menadiol specifications and serves as a tool for batch-level quality control.
Menadiol CoA mostly includes findings from lab analyses of a specific batch. For each Menadiol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Menadiol may be tested according to a variety of international standards, such as European Pharmacopoeia (Menadiol EP), Menadiol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Menadiol USP).
