
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
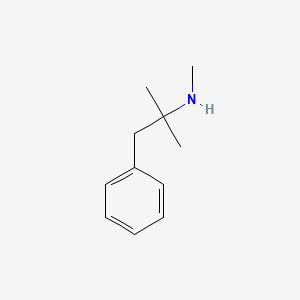

1. Mephentermine Sulfate
2. Mephentermine Sulfate (2:1)
3. Sulfate, Mephentermine
1. Mephenterdrine
2. Mefenterdrin
3. Mephenterdrinum
4. Mephetedrine
5. Mefentermin
6. Vialin
7. N-methylphentermine
8. 100-92-5
9. Wyfentermina
10. Mephine
11. Wyamine
12. N,2-dimethyl-1-phenylpropan-2-amine
13. Mephentermine Sulfate
14. Wy-585
15. 2-methyl-2-methylamino-1-phenylpropane
16. 2-methylamino-2-methyl-1-phenylpropane
17. Mephentermine (inn)
18. N-methyl-omega-phenyl-tert-butylamine
19. Chebi:6755
20. Tez91l71v4
21. Benzeneethanamine, N,.alpha.,.alpha.-trimethyl-
22. Mefentermina
23. Mephenterminum
24. N,.alpha.,.alpha.-trimethylphenethylamine
25. Methyl(2-methyl-1-phenylpropan-2-yl)amine
26. Mephentermine Sodium
27. Phenethylamine, N,.alpha.,.alpha.-trimethyl-
28. Mephentermine [inn]
29. Mephentermine [inn:ban]
30. Mefentermina [inn-spanish]
31. Mephenterminum [inn-latin]
32. N,2-dimethyl-1-phenylpropan-2-amine;n,2-dimethyl-1-phenylpropan-2-amine
33. N-methyl-omega-phenyl-t-butylamine
34. Omega-phenyl-tert-butyl-methylamine
35. N,alpha,alpha-trimethylphenethylamine
36. N,alpha,alpha-trimethylbenzeneethanamine
37. Hsdb 2172
38. Benzeneethanamine, N,alpha,alpha-trimethyl-
39. Ncgc00016570-01
40. Einecs 202-901-6
41. Cas-1212-72-2
42. Brn 1363850
43. Unii-tez91l71v4
44. Phenethylamine, N,alpha,alpha-trimethyl-
45. Fentermin (salt/mix)
46. (1,1-dimethyl-2-phenylethyl)methylamine
47. Spectrum_001611
48. Prestwick0_000726
49. Prestwick1_000726
50. Prestwick2_000726
51. Prestwick3_000726
52. Spectrum2_000474
53. Spectrum3_001220
54. Spectrum4_000188
55. Spectrum5_001008
56. Mephentermine [mi]
57. Mephentermine [hsdb]
58. Bspbio_000652
59. Bspbio_002640
60. Kbiogr_000735
61. Kbioss_002091
62. Mephentermine [vandf]
63. Divk1c_000229
64. Schembl121178
65. Spbio_000608
66. Spbio_002591
67. Mephentermine [who-dd]
68. Bpbio1_000718
69. Gtpl7222
70. Chembl1201234
71. Dtxsid4023256
72. Bdbm81455
73. Kbio1_000229
74. Kbio2_002091
75. Kbio2_004659
76. Kbio2_007227
77. Kbio3_002140
78. Ninds_000229
79. Cas_3677
80. Nsc_3677
81. Zinc8132748
82. .omega.-phenyl-tert-butyl-methylamine
83. N-methyl-.omega.-phenyl-t-butylamine
84. Akos006281398
85. Db01365
86. Idi1_000229
87. N,2-dimethyl-1-phenyl-2-propanamine #
88. N-methyl-.omega.-phenyl-tert-butylamine
89. Ncgc00016570-02
90. Ncgc00016570-03
91. Sbi-0051802.p002
92. Ab00053662
93. N,.alpha.,.alpha.-trimethylbenzeneethanamine
94. C07889
95. D08180
96. Ab00053662_08
97. 100m925
98. N,.alpha.,.alpha.-trimethyl-.beta.-phenethylamine
99. Q6817800
100. Brd-k18194590-065-05-6
| Molecular Weight | 163.26 g/mol |
|---|---|
| Molecular Formula | C11H17N |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 163.136099547 g/mol |
| Monoisotopic Mass | 163.136099547 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 123 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic alpha-Agonists; Adrenergic Agents; Sympathomimetics; Vasoconstrictor Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Mephentermine ... is used to prevent hypotension, which frequently accompanies spinal anesthesia.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 217
IT IS NOT RECOMMENDED FOR ROUTINE USE IN MANAGEMENT OF SHOCK, ESPECIALLY HYPOVOLEMIC SHOCK, ALTHOUGH IT CAN BE GIVEN AS INTERIM DRUG WHILE PREPARATIONS ARE BEING MADE FOR FLUID REPLACEMENT & OTHER MEASURES. /SULFATE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 169
...FREE BASE CAN BE EMPLOYED TOPICALLY AS MYDRIATIC.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 816
For more Therapeutic Uses (Complete) data for MEPHENTERMINE (9 total), please visit the HSDB record page.
Mephentermine may produce arrhythmias, including extrasystoles, and AV block and hypertension. Arrhythmias are most likely to occur in patients with heart disease or those receiving other drugs which may increase cardiac irritability such as cyclopropane or halogenated hydrocarbon general anesthetics. /Mephentermine sulfate/
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 888
The CNS stimulating effects of mephentermine may result in nervousness, anxiety, seizures, or tachycardia. Large overdosage of the drug has caused visual hallucinations of colored geometric forms, paranoid psychosis, and euphoria. Drowsiness, weeping, incoherence, weakness, numbness, and tingling of the extremities have been reported. Withdrawal of the drug results in rapid disappearance of adverse CNS effects. /Mephentermine sulfate/
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 888
AS THE FREE BASE IS CURRENTLY USED, UNTOWARD EFFECTS ARE VERY UNCOMMON.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 816
MEPHENTERMINE MAY INCR UTERINE CONTRACTIONS ESPECIALLY DURING THE THIRD TRIMESTER OF PREGNANCY &, THEREFORE, SHOULD NOT BE USED IN PREGNANT WOMEN UNLESS THE POTENTIAL BENEFITS OUTWEIGH THE POSSIBLE RISKS. /MEPHENTERMINE SULFATE/
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 889
For more Drug Warnings (Complete) data for MEPHENTERMINE (10 total), please visit the HSDB record page.
Used to maintain blood pressure in hypotensive states.
Mephentermine is a sympathomimetic agent with mainly indirect effects on adrenergic receptors. It is used to maintain blood pressure in hypotensive states, for example, following spinal anesthesia. Although the central stimulant effects of mephentermine are much less than those of amphetamine, its use may lead to amphetamine-type dependence. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1248)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
Adrenergic alpha-1 Receptor Agonists
Compounds that bind to and activate ADRENERGIC ALPHA-1 RECEPTORS. (See all compounds classified as Adrenergic alpha-1 Receptor Agonists.)
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CA - Adrenergic and dopaminergic agents
C01CA11 - Mephentermine
...MEPHENTERMINE.../IS/ WELL ABSORBED FROM DIGESTIVE TRACT. .../IT/ MAY BE ABSORBED TO A GREATER OR LESSER EXTENT FROM NASAL MUCOSA.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 108
THE MAJORITY OF THE DRUG IS EXCRETED IN THE URINE WITHIN 24 HR. MEPHENTERMINE IS REABSORBED IN THE RENAL TUBULES. EXCRETION OF THE DRUG & ITS METABOLITES IS MORE RAPID IN ACIDIC URINE.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 888
Hepatic, by N-demethylation and then p-hydroxylation.
MEPHENTERMINE IS METABOLIZED IN THE LIVER BY N-DEMETHYLATION & SUBSEQUENT P-HYDROXYLATION TO NORMEPHENTERMINE & P-HYDROXYNORMEPHENTERMINE. /SULFATE/
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 888
MEPHENTERMINE WAS METABOLIZED IN VITRO BY RABBIT LIVER MICROSOMES TO PHENTERMINE (II), N-HYDROXYMEPHENTERMINE (III), & N-HYDROXYPHENTERMINE. METABOLITES (II) & (III) PLUS UNCHANGED MEPHENTERMINE FOUND IN URINE OF HUMAN ADMIN SINGLE DOSE OF MEPHENTERMINE SULFATE.
PMID:2665 BECKETT AH, BELANGER PM; J PHARM PHARMACOL 27 (12): 928 (1975)
Metabolites of mephentermine (MP), phentermine (Ph), p-hydroxy-MP, p-hydroxy-Ph, N-hydroxy-MP and N-hydroxy-Ph on incubation with rat liver microsomal and cytosolic preparations were identified by glc and glc mass spectrometry. Identification of the metabolites indicated the following new metabolic routes of MP: NADPH dependent microsomal formation of p-hydroxy-MP from MP, of p-hydroxy-Ph from p-hydroxy-MP, and the NADH-dependent microsomal formation of Ph from N-hydroxy-Ph.
PMID:1523866 Mori MA, et al; Xenobiotica 22 (4):451-7 (1992)
The urinary excretion of mephentermine (I) and its major metabolite phentermine (II) in human volunteers over a period of several days after oral admin of the drug is described. The total proportion of the drug excreted during 54 hr was 57 to 83%. The ingestion of acetazolamide shortly after admin of I resulted in decr in excretion of both I and II during one day. The administration of furosemide only produced a urinary diluting effect during 2-4 hr after admin.
PMID:16867696 Delbeke FT, Debackers M; J Pharm Biomed Anal 3 (2): 141-8 (1985)
17 to 18 hours.
17 to 18 hours
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2743
Mephentermine is an alpha adrenergic receptor agonist, but also acts indirectly by releasing endogenous norepinephrine. Cardiac output and systolic and diastolic pressures are usually increased. A change in heart rate is variable, depending on the degree of vagal tone. Sometimes the net vascular effect may be vasodilation. Large doses may depress the myocardium or produce central nervous system (CNS) effects.
Mephentermine is a sympathomimetic drug that acts both directly and indirectly; it has many similarities to ephedrine. After an intramuscular injection, the onset of action is prompt (within 5 to 15 minutes), and effects may last for several hours.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 216
Since the drug releases norepinephrine, cardiac contraction is enhanced and cardiac output and systolic and diastolic pressures are usually increased. The change in heart rate is variable, depending on the degree of vagal tone.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 216

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
85
PharmaCompass offers a list of Mephentermine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mephentermine manufacturer or Mephentermine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mephentermine manufacturer or Mephentermine supplier.
PharmaCompass also assists you with knowing the Mephentermine API Price utilized in the formulation of products. Mephentermine API Price is not always fixed or binding as the Mephentermine Price is obtained through a variety of data sources. The Mephentermine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mephentermine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mephentermine, including repackagers and relabelers. The FDA regulates Mephentermine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mephentermine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Mephentermine supplier is an individual or a company that provides Mephentermine active pharmaceutical ingredient (API) or Mephentermine finished formulations upon request. The Mephentermine suppliers may include Mephentermine API manufacturers, exporters, distributors and traders.
click here to find a list of Mephentermine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mephentermine DMF (Drug Master File) is a document detailing the whole manufacturing process of Mephentermine active pharmaceutical ingredient (API) in detail. Different forms of Mephentermine DMFs exist exist since differing nations have different regulations, such as Mephentermine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mephentermine DMF submitted to regulatory agencies in the US is known as a USDMF. Mephentermine USDMF includes data on Mephentermine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mephentermine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mephentermine suppliers with USDMF on PharmaCompass.
Mephentermine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mephentermine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mephentermine GMP manufacturer or Mephentermine GMP API supplier for your needs.
A Mephentermine CoA (Certificate of Analysis) is a formal document that attests to Mephentermine's compliance with Mephentermine specifications and serves as a tool for batch-level quality control.
Mephentermine CoA mostly includes findings from lab analyses of a specific batch. For each Mephentermine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mephentermine may be tested according to a variety of international standards, such as European Pharmacopoeia (Mephentermine EP), Mephentermine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mephentermine USP).
