

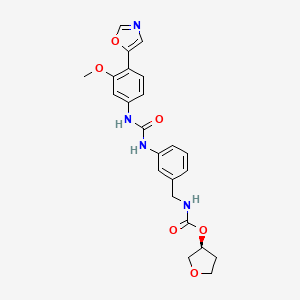

1. N-3-(3-(3-methoxy-4-oxazol-5-ylphenyl)ureido)benzylcarbamic Acid Tetrahydrofuran-3-yl Ester
2. Vx 497
3. Vx-497
1. 198821-22-6
2. Vx-497
3. Mmpd
4. Vi-21497
5. 2zl2ba06fu
6. (s)-tetrahydrofuran-3-yl 3-(3-(3-methoxy-4-(oxazol-5-yl)phenyl)ureido)benzylcarbamate
7. Vi-21,497
8. Chembl304087
9. [(3s)-oxolan-3-yl] N-[[3-[[3-methoxy-4-(1,3-oxazol-5-yl)phenyl]carbamoylamino]phenyl]methyl]carbamate
10. Vx497
11. Merimepodib, Vi-21497, Vx-497
12. (s)-tetrahydro-3-furyl (m-(3-(3-methoxy-4-(5-oxazolyl)phenyl)ureido)benzyl)carbamate
13. Carbamic Acid, ((3-((((3-methoxy-4-(5-oxazolyl)phenyl)amino)carbonyl)amino)phenyl)methyl)-, (3s)-tetrahydro-3-furanyl Ester
14. Vx 497
15. Merimepodib [usan:inn]
16. Unii-2zl2ba06fu
17. Merimebodib
18. Carbamic Acid, [[3-[[[[3-methoxy-4-(5-oxazolyl)phenyl]amino]carbonyl]amino]phenyl]methyl]-, (3s)-tetrahydro-3-furanyl Ester
19. Merimepodib [usan]
20. Merimepodib [mi]
21. Merimepodib [inn]
22. Merimepodib (usan/inn)
23. Merimepodib [who-dd]
24. Schembl329922
25. Gtpl10741
26. Dtxsid70173639
27. [[3-[[[[3-methoxy-4-(5-oxazolyl)phenyl]amino]carbonyl]amino]phenyl]methyl]carbamic Acid (3s)-tetrahydro-3-furanyl Ester
28. Bcp28336
29. Ex-a1201
30. Yha82122
31. Zinc3975663
32. Bdbm50102249
33. Fd5020
34. Mfcd09837807
35. S6689
36. Db04862
37. N-3-(3-(3-methoxy-4-oxazol-5-ylphenyl)ureido)benzylcarbamic Acid Tetrahydrofuran-3-yl Ester
38. Ncgc00378589-01
39. Ac-35396
40. As-75124
41. Hy-13986
42. D04936
43. A919457
44. Q1727082
45. (3s)-oxolan-3-yl N-{[3-({[3-methoxy-4-(1,3-oxazol-5-yl)phenyl]carbamoyl}amino)phenyl]methyl}carbamate
46. [(3s)-tetrahydrofuran-3-yl] N-[[3-[(3-methoxy-4-oxazol-5-yl-phenyl)carbamoylamino]phenyl]methyl]carbamate
47. {3-[3-(3-methoxy-4-oxazol-5-yl-phenyl)-ureido]-benzyl}-carbamic Acid (s)-(tetrahydro-furan-3-yl) Ester
48. {3-[3-(3-methoxy-4-oxazol-5-yl-phenyl)-ureido]-benzyl}-carbamic Acid Tetrahydro-furan-3-yl Ester
49. Carbamic Acid, N-[[3-[[[[3-methoxy-4-(5-oxazolyl)phenyl]amino]carbonyl]amino]phenyl]methyl]-, (3s)-tetrahydro-3-furanyl Ester
50. N-[3-3-[3-methoxy-4-(5-oxazolyl)phenyl]ureido]benzyl]carbamic Acid Tetrahydrofuran-3-(s)-yl Ester
| Molecular Weight | 452.5 g/mol |
|---|---|
| Molecular Formula | C23H24N4O6 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 452.16958450 g/mol |
| Monoisotopic Mass | 452.16958450 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 652 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of hepatitis C virus (HCV) infection.
Merimepodib, an oral drug, contains a novel inhibitor of inosine monophosphate dehydrogenase (IMPDH), an enzyme responsible for stimulating the production of lymphocytes. Merimepodib has the potential to exert direct antiviral activity, as well as affect the immune response by acting on lymphocyte migration and proliferation. Consequently, merimepodib may be an effective treatment for hepatitis C virus (HCV) infection, as the disease involves both viral proliferation and liver inflammation.
Merimepodib is a orally active inhibitor of inosine monophospate dehydrogenase (IMPDH). IMPDH inhibition leads to a reduction in intracellular guanosine triphosphate (GTP), a molecule required for DNA and RNA synthesis.

LOOKING FOR A SUPPLIER?