
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 5 Aminosalicylate
2. 5 Aminosalicylic Acid
3. 5-aminosalicylate
4. 5-aminosalicylic Acid
5. Asacol
6. Asacolon
7. Ascolitin
8. Canasa
9. Claversal
10. Fivasa
11. Hydrochloride, Mesalamine
12. Lixacol
13. M Aminosalicylic Acid
14. M-aminosalicylic Acid
15. Mesalamine Hydrochloride
16. Mesalamine Monosodium Salt
17. Mesalazine
18. Mesasal
19. Meta Aminosalicylic Acid
20. Meta-aminosalicylic Acid
21. Monosodium Salt, Mesalamine
22. Novo 5 Asa
23. Novo-5 Asa
24. Pentasa
25. Rowasa
26. Salofalk
1. 5-aminosalicylic Acid
2. Mesalazine
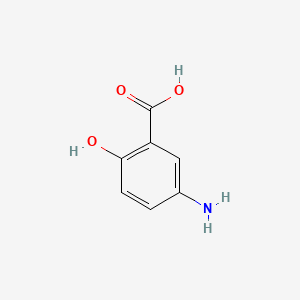
3. 89-57-6
4. 5-amino-2-hydroxybenzoic Acid
5. Asacol
6. Pentasa
7. Canasa
8. 5-asa
9. Claversal
10. Rowasa
11. Salofalk
12. M-aminosalicylic Acid
13. Lialda
14. Mesasal
15. Benzoic Acid, 5-amino-2-hydroxy-
16. Fisalamine
17. Apriso
18. Lixacol
19. Sfrowasa
20. P-aminosalicylsaeure
21. Asacolitin
22. Mesalazina
23. Mesalazinum
24. Iialda
25. 5-amino-2-hydroxy-benzoic Acid
26. Asacol Hd
27. Mesalamine [usan]
28. 5-amino Salicylic Acid
29. Salicylic Acid, 5-amino-
30. 2-hydroxy-5-aminobenzoic Acid
31. Max-002
32. 3-carboxy-4-hydroxyaniline
33. Mfcd00007877
34. Nsc 38877
35. Nsc-38877
36. Mesalazine [inn]
37. Mls001424012
38. 4q81i59gxc
39. Chebi:6775
40. Mesalamine (usan)
41. 5-?aminosalicylic Acid (mesalazine)
42. Cas-89-57-6
43. Ncgc00016344-03
44. Mesalazinum [latin]
45. Smr000145728
46. Dsstox_cid_4506
47. Dsstox_rid_77435
48. Dsstox_gsid_24506
49. Mesalazina [spanish]
50. P-aminosalicylsaeure [german]
51. Mesavancol
52. Delzicol
53. Mesavance
54. Mezavant
55. Mesalazine Mmx
56. Mezavant Xl
57. Mesalamine (usp)
58. Pentasa (tn)
59. Salofalk Granu-stix
60. Apriso (tn)
61. Asacol (tn)
62. Canasa (tn)
63. Lialda (tn)
64. Rowasa (tn)
65. 5-as
66. Ccris 7334
67. Sr-01000763486
68. Mesalamine [usan:usp]
69. Einecs 201-919-1
70. Brn 2090421
71. Unii-4q81i59gxc
72. Ai3-15564
73. Hsdb 7512
74. Ajg-501
75. Spd 476
76. Spd-476
77. Spd-480
78. Mesalamine (tn)
79. Delzicol (tn)
80. Sfrowasa (tn)
81. Mesalamine (lialda)
82. 5-aminosalicylic_acid
83. Md-0901
84. 5-aminosalicyclic Acid
85. 5-amino-salicylic Acid
86. Mesalamine [mi]
87. Mesalazine [jan]
88. Prestwick0_001069
89. Prestwick1_001069
90. Prestwick2_001069
91. Prestwick3_001069
92. Mesalamine [hsdb]
93. Wln: Zr Dq Cvq
94. Z-206
95. Mesalamine [vandf]
96. Chembl704
97. Mesalazine (jp17/inn)
98. Ec 201-919-1
99. Cid_4075
100. Mesalazine [mart.]
101. Mesalamine [usp-rs]
102. Mesalazine [who-dd]
103. Oprea1_847633
104. Schembl31297
105. 3amino-6-hydroxybenzoic Acid
106. Bspbio_001058
107. Kbiogr_002425
108. Kbioss_002431
109. 4-14-00-02058 (beilstein Handbook Reference)
110. Mls000758287
111. 5-aminosalicylic Acid, 95%
112. 5-aminosalicylic Acid, Tablet
113. Bidd:gt0811
114. 3-amino-6-hydroxybenzoic Acid
115. Spbio_002969
116. Bpbio1_001164
117. Gtpl2700
118. Zinc1688
119. 5-amino 2-hydroxy Benzoic Acid
120. Dtxsid5024506
121. Mesalamine [orange Book]
122. Mesalazine [ep Impurity]
123. Schembl18038934
124. 5-aminosalicylic Acid, >=99%
125. Bdbm60918
126. Kbio2_002425
127. Kbio2_004993
128. Kbio2_007561
129. Kbio3_002904
130. Mesalazine [ep Monograph]
131. Cmap_000045
132. Hms1571e20
133. Hms2051m21
134. Hms2090i09
135. Hms2098e20
136. Hms3393m21
137. Hms3649k15
138. Hms3651m15
139. Hms3715e20
140. Mesalamine [usp Monograph]
141. Pharmakon1600-01505993
142. Bcp05326
143. Nsc38877
144. Tox21_110384
145. Tox21_201610
146. Tox21_303125
147. Ac8101
148. Bbl013046
149. Nsc759301
150. S1681
151. Stk301678
152. Akos000118959
153. Tox21_110384_1
154. Ac-2764
155. Bcp9000175
156. Ccg-100829
157. Db00244
158. Hs-0100
159. Nc00079
160. Nsc-759301
161. Ncgc00016344-01
162. Ncgc00016344-02
163. Ncgc00016344-04
164. Ncgc00016344-05
165. Ncgc00016344-07
166. Ncgc00090934-01
167. Ncgc00090934-02
168. Ncgc00257142-01
169. Ncgc00259159-01
170. 5-amino-2-hydroxobenzoic Acid Monohydrate
171. Bp-13074
172. Hy-15027
173. Sy002854
174. 5-aminosalicylic Acid, Analytical Standard
175. A0317
176. Ab00374979
177. Am20060091
178. Ft-0619950
179. Sw197303-4
180. C07138
181. D00377
182. Ab00374979-09
183. Ab00374979-10
184. Ab00374979_11
185. Ab00374979_12
186. Q412479
187. 5-amino-2-hydroxybenzoic Acid,5-aminosalicylic Acid
188. Q-201355
189. Sr-01000763486-3
190. Sr-01000763486-4
191. Sr-01000763486-9
192. Z57127471
193. F1918-0003
194. Mesalazine, European Pharmacopoeia (ep) Reference Standard
195. Mesalamine, United States Pharmacopeia (usp) Reference Standard
196. Mesalamine, Pharmaceutical Secondary Standard; Certified Reference Material
197. Mesalazine For System Suitability, European Pharmacopoeia (ep) Reference Standard
198. 51481-17-5
| Molecular Weight | 153.14 g/mol |
|---|---|
| Molecular Formula | C7H7NO3 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 153.042593085 g/mol |
| Monoisotopic Mass | 153.042593085 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 20 | |
|---|---|
| Drug Name | Apriso |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each APRISO capsule is a delayed- and extended-release dosage form for oral administration. Each capsule contains 0.375 g of mesalamine USP (5-aminosalicylic acid, 5-ASA), an anti-inflammatory drug. The structural formula of mesalamine is: Molecula... |
| Active Ingredient | Mesalamine |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 375mg |
| Market Status | Prescription |
| Company | Salix Pharms |
| 2 of 20 | |
|---|---|
| Drug Name | Asacol |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each Asacol delayed release tablet for oral administration contains 400mg of mesalamine, an anti-inflammatory drug. The Asacol delayed-release tablets are coated with acrylic based resin, Eudragit S (methacrylic acid copolymer B, NF), which dissolves... |
| Active Ingredient | Mesalamine |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 3 of 20 | |
|---|---|
| Drug Name | Asacol hd |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each Asacol HD delayed-release tablet for oral administration contains 800 mg of mesalamine, an aminosalicylate. Asacol HD delayed-release tablets have an outer protective coat consisting of a combination of acrylic based resins, Eudragit S (methacry... |
| Active Ingredient | Mesalamine |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 800mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 4 of 20 | |
|---|---|
| Drug Name | Canasa |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | The active ingredient in CANASA 1000 mg rectal suppositories is mesalamine, also known as mesalazine or 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid, and is classified as an anti-inflammatory drug. Each CANAS... |
| Active Ingredient | Mesalamine |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 1gm |
| Market Status | Prescription |
| Company | Forest Labs |
| 5 of 20 | |
|---|---|
| Drug Name | Delzicol |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each DELZICOL (mesalamine) delayed-release capsule for oral administration contains 400 mg of mesalamine, an aminosalicylate. DELZICOL (mesalamine) delayed-release capsules contain acrylic based resin, Eudragit S (methacrylic acid copolymer type B, N... |
| Active Ingredient | Mesalamine |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 6 of 20 | |
|---|---|
| Drug Name | Lialda |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each LIALDA delayed-release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is:Molecul... |
| Active Ingredient | Mesalamine |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 1.2gm |
| Market Status | Prescription |
| Company | Shire |
| 7 of 20 | |
|---|---|
| Drug Name | Mesalamine |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | The active ingredient in ROWASA (mesalamine) Rectal Suspension Enema, a disposable (60 mL) unit, is mesalamine, also known as 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid.The empirical formula is C7H7NO3, r... |
| Active Ingredient | Mesalamine |
| Dosage Form | Enema |
| Route | Rectal |
| Strength | 4gm/60ml |
| Market Status | Prescription |
| Company | Teva; Perrigo Israel |
| 8 of 20 | |
|---|---|
| Drug Name | Pentasa |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | PENTASA (mesalamine) for oral administration is a controlled-release formulation of mesalamine, an aminosalicylate anti-inflammatory agent for gastrointestinal use.Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid. It has a molecular weight of... |
| Active Ingredient | Mesalamine |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Shire |
| 9 of 20 | |
|---|---|
| Drug Name | Rowasa |
| Drug Label | The active ingredient in ROWASA (mesalamine) Rectal Suspension Enema, a disposable (60 mL) unit, is mesalamine, also known as 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid.The empirical formula is C7H7NO3, r... |
| Active Ingredient | Mesalamine |
| Dosage Form | Enema |
| Route | Rectal |
| Strength | 4gm/60ml |
| Market Status | Prescription |
| Company | Meda Pharms |
| 10 of 20 | |
|---|---|
| Drug Name | Sfrowasa |
| Active Ingredient | Mesalamine |
| Dosage Form | Enema |
| Route | Rectal |
| Strength | 4gm/60ml |
| Market Status | Prescription |
| Company | Meda Pharms |
| 11 of 20 | |
|---|---|
| Drug Name | Apriso |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each APRISO capsule is a delayed- and extended-release dosage form for oral administration. Each capsule contains 0.375 g of mesalamine USP (5-aminosalicylic acid, 5-ASA), an anti-inflammatory drug. The structural formula of mesalamine is: Molecula... |
| Active Ingredient | Mesalamine |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 375mg |
| Market Status | Prescription |
| Company | Salix Pharms |
| 12 of 20 | |
|---|---|
| Drug Name | Asacol |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each Asacol delayed release tablet for oral administration contains 400mg of mesalamine, an anti-inflammatory drug. The Asacol delayed-release tablets are coated with acrylic based resin, Eudragit S (methacrylic acid copolymer B, NF), which dissolves... |
| Active Ingredient | Mesalamine |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 13 of 20 | |
|---|---|
| Drug Name | Asacol hd |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each Asacol HD delayed-release tablet for oral administration contains 800 mg of mesalamine, an aminosalicylate. Asacol HD delayed-release tablets have an outer protective coat consisting of a combination of acrylic based resins, Eudragit S (methacry... |
| Active Ingredient | Mesalamine |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 800mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 14 of 20 | |
|---|---|
| Drug Name | Canasa |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | The active ingredient in CANASA 1000 mg rectal suppositories is mesalamine, also known as mesalazine or 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid, and is classified as an anti-inflammatory drug. Each CANAS... |
| Active Ingredient | Mesalamine |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 1gm |
| Market Status | Prescription |
| Company | Forest Labs |
| 15 of 20 | |
|---|---|
| Drug Name | Delzicol |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each DELZICOL (mesalamine) delayed-release capsule for oral administration contains 400 mg of mesalamine, an aminosalicylate. DELZICOL (mesalamine) delayed-release capsules contain acrylic based resin, Eudragit S (methacrylic acid copolymer type B, N... |
| Active Ingredient | Mesalamine |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 16 of 20 | |
|---|---|
| Drug Name | Lialda |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | Each LIALDA delayed-release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is:Molecul... |
| Active Ingredient | Mesalamine |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 1.2gm |
| Market Status | Prescription |
| Company | Shire |
| 17 of 20 | |
|---|---|
| Drug Name | Mesalamine |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | The active ingredient in ROWASA (mesalamine) Rectal Suspension Enema, a disposable (60 mL) unit, is mesalamine, also known as 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid.The empirical formula is C7H7NO3, r... |
| Active Ingredient | Mesalamine |
| Dosage Form | Enema |
| Route | Rectal |
| Strength | 4gm/60ml |
| Market Status | Prescription |
| Company | Teva; Perrigo Israel |
| 18 of 20 | |
|---|---|
| Drug Name | Pentasa |
| PubMed Health | Mesalamine |
| Drug Classes | Anti-Inflammatory, Gastrointestinal Agent |
| Drug Label | PENTASA (mesalamine) for oral administration is a controlled-release formulation of mesalamine, an aminosalicylate anti-inflammatory agent for gastrointestinal use.Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid. It has a molecular weight of... |
| Active Ingredient | Mesalamine |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Shire |
| 19 of 20 | |
|---|---|
| Drug Name | Rowasa |
| Drug Label | The active ingredient in ROWASA (mesalamine) Rectal Suspension Enema, a disposable (60 mL) unit, is mesalamine, also known as 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid.The empirical formula is C7H7NO3, r... |
| Active Ingredient | Mesalamine |
| Dosage Form | Enema |
| Route | Rectal |
| Strength | 4gm/60ml |
| Market Status | Prescription |
| Company | Meda Pharms |
| 20 of 20 | |
|---|---|
| Drug Name | Sfrowasa |
| Active Ingredient | Mesalamine |
| Dosage Form | Enema |
| Route | Rectal |
| Strength | 4gm/60ml |
| Market Status | Prescription |
| Company | Meda Pharms |
Mesalamine rectal suspension is indicated for the treatment of mild to moderate distal ulcerative colitis, proctosigmoiditis, and proctitis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Mesalamine rectal suspension is indicated to help maintain remission of distal ulcerative colitis. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Mesalamine suppositories are indicated for the treatment of active ulcerative proctitis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Mesalamine is indicated to treat and to maintain remission of mild to moderate ulcerative colitis or (Crohn's disease). /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1931
Lialda /the first oral once daily mesalamine tablet/ is indicated for the induction of remission in patients with active, mild to moderate ulcerative colitis.
FDA: Center for Drug Evaluation and Research (CDER); Label Information for Lialda (Mesalamine) Lasted updated 2007. Available from, as of March 30, 2007: https://www.fda.gov/cder/foi/label/2007/022000lbl.pdf
Oral and rectal mesalamine preparations usually are well tolerated. The most common adverse effects of oral or rectal mesalamine are GI effects and headache. In clinical studies, most adverse effects associated with oral or rectal preparations were mild in severity and were transient or reversible. However, adverse effects have been severe enough to require discontinuance of the drug in less than 1% or in up to about 4-5% of patients receiving rectal or oral mesalamine, respectively, although in some studies, the rate of discontinuance of the drug was similar to or less than in those receiving placebo. Most of the adverse effects reported with the use of oral mesalamine delayed-release tablets were similar in short- and long-term studies.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3013
Exacerbation of colitis symptoms was reported in 3% of patients receiving oral mesalamine delayed-release tablets. Other adverse GI effects associated with oral mesalamine extended-release capsules and occurring in less than 1% of patients, include abdominal distention, constipation, duodenal ulcer, dysphagia, eructation, esophageal or mouth ulcer, fecal incontinence, GI bleeding (e.g., rectal bleeding), stool abnormalities (e.g., change in color or texture), oral moniliasis, and thirst, although a causal relationship to the drug of many of these adverse effects has not been established.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3013
In controlled clinical trials in patients receiving oral mesalamine delayed-release tablets, abdominal pain, eructation, nausea, diarrhea, dyspepsia, vomiting, constipation, flatulence, exacerbation of colitis, abdominal enlargement, gastroenteritis, GI hemorrhage, rectal disorder (e.g., hemorrhage, tenesmus), and stool abnormalities, were the most common adverse GI effects, occurring in about 2-18% of patients; dry mouth, indigestion, stomatitis, and cramping were reported rarely. Frequency of these GI effects did not seem to increase with increased dosages, although in uncontrolled studies, the incidence of abdominal pain, flatulence, and GI bleeding were dose related. The most common adverse GI effects of oral mesalamine extended-released capsules were diarrhea (including melena), nausea, abdominal pain, dyspepsia, vomiting, anorexia, worsening of ulcerative colitis, and rectal urgency, occurring in greater than 0.4-3% of patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3013
An acute intolerance syndrome (sensitivity reaction), characterized by cramping, abdominal pain, bloody diarrhea, and, occasionally, fever, headache, malaise, conjunctivitis, pruritus, and rash, has occurred in a few patients receiving mesalamine and required prompt discontinuance of the drug. In patients manifesting such intolerance, a history of sulfasalazine intolerance, if any, should be reevaluated.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3014
For more Drug Warnings (Complete) data for MESALAMINE (26 total), please visit the HSDB record page.
Mesalazine is indicated for the induction of remission in patients with active or mild to moderate acute exacerbations of ulcerative colitis and for the maintenance of remission of ulcerative colitis,. Prescribing information for mesalazine in the UK also indicates the medication for the maintenance of remission of Crohn's ileo-colitis.
FDA Label
Mesalazine is one of the two components of sulphasalazine, the other being sulphapyridine. It is the latter which is responsible for the majority of the side effects associated with sulphasalazine therapy whilst mesalazine is known to be the active moiety in the treatment of ulcerative colitis. The pharmacodynamic actions of mesalazine occur in the colonic/rectal mucosae local to the delivery of drug from mesalazine tablets into the lumen. There is information suggesting that the severity of colonic inflammation in ulcerative colitis patients treated with mesalazine is inversely correlated with mucosal concentrations of mesalazine. Plasma concentrations representing systemically absorbed mesalazine are not believed to contribute extensively to efficacy.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
A07EC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07E - Intestinal antiinflammatory agents
A07EC - Aminosalicylic acid and similar agents
A07EC02 - Mesalazine
Absorption
Depending on the formulation administered, prescribing information for orally administered delayed-released tablets of 2.4g or 4.8g of mesalazine given once daily for 14 days to healthy volunteers was to found to be about 21% to 22% of the administered dose while prescribing information for an orally administered controlled-release capsule formulation suggests 20% to 30% of the mesalazine in the formulation is absorbed. In contrast, when mesalamine is administered orally as an unformulated 1-g aqueous suspension, mesalazine is approximately 80% absorbed.
Route of Elimination
Elimination of mesalazine is mainly via the renal route following metabolism to N-acetyl-5-aminosalicylic acid (acetylation). However, there is also limited excretion of the parent mesalazine drug in the urine. After the oral administration of the extended-release formulation of mesalazine, of the approximately 21% to 22% of the drug absorbed, less than 8% of the dose was excreted unchanged in the urine after 24 hours, compared with greater than 13% for N-acetyl-5-aminosalicylic acid. When given the controlled-release formulation, about 130 mg free mesalazine was recovered in the feces following a single 1-g dose, which was comparable to the 140 mg of mesalazine recovered from the molar equivalent sulfasalazine tablet dose of 2.5 g F3001]. Elimination of free mesalazine and salicylates in feces increased proportionately with the dose given. N-acetylmesalazine was the primary compound excreted in the urine (19% to 30%) following the controlled-release dosing.
Volume of Distribution
The apparent volume of distribution (Vd) of the drug in adults is approximately 0.2 L/kg.
Clearance
The mean (SD) renal clearance in L/h for mesalazine following the single dose administration of mesalazine delayed-release tablets 4.8g under fasting conditions to young and elderly subjects was documented as 2.05 (1.33) in young subjects aged 18 to 35 years old, 2.04 (1.16) in elderly subjects aged 65 to 75 years old, and 2.13 (1.20) in elderly subjects older than 75 years.
Following oral administration, low concentrations of mesalamine and higher concentrations of its metabolite, N-acetyl-5-aminosalicylic acid, have been detected in human breast milk. It is not known whether mesalamine or its metabolites are distributed into milk in humans following rectal administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
Mesalamine and N-acetyl-5-aminosalicylic acid cross the placenta following oral administration; however, serum concentrations of mesalamine in umbilical cord and amniotic fluid are very low. It is not known whether mesalamine crosses the placenta following rectal administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
In vitro, mesalamine and N-acetyl-5-aminosalicylic acid are approximately 44-55 and 80% bound, respectively, to plasma proteins. Protein binding of N-acetyl-5-aminosalicylic acid does not appear to be concentration dependent at concentrations ranging from 1-10 ug/mL.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
It is generally accepted that 5-aminosalicylate (5-ASA; mesalamine), widely used in inflammatory bowel disease therapy, exerts its action from the intraluminal site of the intestine. In addition to local metabolism of 5-ASA, it has been assumed that therapeutic mucosal concentrations of 5-ASA depend on transporter-mediated secretion back to the lumen. ... The hypothesis that 5-ASA represents a substrate of P-glycoprotein (P-gp) and/or multidrug resistance-associated protein 2 (MRP2), thereby possibly contributing to variable therapeutic effects /was tested/. Polarized, basal-to-apical transport of [(3)H]5-ASA was studied in monolayers of Caco-2 and L-MDR cells, both of which express P-gp in their apical membrane, as well as in MDCK cells transfected with human MRP2. Moreover, we investigated the influence of 5-ASA on transport of digoxin in Caco-2 cells. In Caco-2 cells a P-gp-mediated efflux of 5-ASA (5-500 muM) could be excluded. Likewise, in L-MDR1 and MRP2 cells no transport differences in either the basal-to-apical or apical-to-basal direction were measurable. 5-ASA (50 muM to 5 mM) had no effect on the transport of digoxin. ...
PMID:16944117 Xin HW et al; Eur J Clin Pharmacol 62 (10): 871-875 (2006)
For more Absorption, Distribution and Excretion (Complete) data for MESALAMINE (15 total), please visit the HSDB record page.
The primary metabolite of mesalazine (5-aminosalicylic acid) is predominantly N-acetyl-5-aminosalicylic acid (Ac-5-ASA). This metabolite is generated via N-acetyltransferase (NAT) activity in the liver and intestinal mucosa cells, largely by NAT-1, in particular.
Mesalazine (5-aminosalicylic acid, 5-ASA), an anti-inflammatory agent for the treatment of inflammatory bowel diseases, is metabolized in organism to the principal biotransformation product, N-acetyl-5-ASA. Some other phase II metabolites (N-formyl-5-ASA, N-butyryl-5-ASA, N-beta-d-glucopyranosyl-5-ASA) have also been described. 5-ASA is a polar compound and besides it exhibits amphoteric properties. ...
PMID:16466733 Nobilis M et al; J Chromatogr A 1119 (1-2): 299-308 (2006)
The exact metabolic fate of mesalamine has not been clearly established. The drug undergoes rapid N-acetylation, probably in the liver, to form N-acetyl-5-aminosalicylic acid; mesalamine and N-acetyl-5-aminosalicylic acid also may undergo conjugation with glucuronic acid. Several other, unidentified metabolites also may be formed. It has been suggested that N-acetylation also may occur (to a limited extent) in the intestinal wall and/or the lumen. The intestinal flora probably are involved in this acetylation, and extensive floral acetylation may adversely affect clinical efficacy of the drug. Correlation between acetylator phenotype of patients receiving mesalamine and the degree of N-acetylation does not appear to exist. Although it has been suggested that N-acetyl-5-aminosalicylic acid may be pharmacologically active, therapeutic response has been poor in some patients treated rectally with this metabolite, and the relative contribution of this metabolite to the therapeutic effect of mesalamine remains questionable. N-Acetyl-5-aminosalicylic acid did not inhibit lipoxygenase in vitro.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
Mesalazine has known human metabolites that include mesalazine, N-acetyl.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The apparent elimination half-life documented for oral delayed-release mesalazine tablets is 7 to 12 hours. The elimination half-life recorded for the active N-acetyl-5-aminosalicylic acid metabolite generated from the administration of oral delayed-release mesalazine tablets is 12 to 23 hours.
Elimination of metabolite: 5 to 10 hr /N-acetyl-5-aminosalicylci acid/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Elimination: 0.5-1.5 hours
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Although the mechanism of action of mesalazine is not fully understood, it is believed to possess a topical anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalazine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon. Furthermore, mesalazine also has the potential to inhibit the activation of Nuclear Factor kappa B (NKkB) and consequently the production of key of pro-inflammatory cytokines. It has been proposed that reduced expression of PPAR gamma nuclear receptors (gamma form of peroxisome proliferator-activated receptors) may be implicated in ulcerative colitis. There is evidence that mesalazine produces pharmacodynamic effects through direct activation of PPAR gamma receptors in the colonic/rectal epithelium as well. Moreover, since increased leukocyte migration, abnormal cytokine production, increased production of arachidonic acid metabolites, particularly leukotriene B4, and increased free radical formation in the inflamed intestinal tissue are all present in patients with inflammatory bowel disease it is also believed that mesalazine has in-vitro and in-vivo pharmacological effects that inhibit leukocyte chemotaxis, decrease cytokine and leukotriene production and scavenge for free radicals.
Mesalamine is effective in the treatment of inflammatory bowel diseases. However, the mechanisms of action of mesalamine remain unclear. IEC-6 and IRD-98, nontransformed rat small intestinal epithelial cell lines, were used to examine the effect of mesalamine on the expression of manganese superoxide dismutase (MnSOD). Rats were given mesalamine enemas to determine the effect on colonic MnSOD expression. Treatment with mesalamine at 0.02 or 2 mg/mL induced MnSOD mRNA levels 2.67-fold or 5.66-fold, respectively. Inhibition of 5-lipoxygenase activating protein with MK-886 or cyclooxygenase with indomethacin did not influence the level of MnSOD mRNA. Nuclear run-on experiments demonstrated an increase in de novo transcription following treatment with mesalamine. MnSOD protein levels were induced 2-fold at 24 hr and 4.23-fold at 48 hr following treatment with 1 mg/mL mesalamine. Mesalamine increased MnSOD 1.7-fold in vivo. Pretreatment with mesalamine significantly protected IRD-98 cells from tumor necrosis factor-alpha cytotoxicity. This is the first example of transcriptional gene regulation by mesalamine. The induction of MnSOD by mesalamine may contribute to the therapeutic mechanism of mesalamine.
PMID:11557525 Valentine JF; Am J Physiol Gastrointest Liver Physiol 281 (4): G1044-50 (2001)
Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with inflammatory bowel disease. Mesalamine appears to diminish inflammation by inhibiting cyclooxygenase and lipoxygenase, thereby decreasing the production of prostaglandins, and leukotrienes and hydroxyeicosatetraenoic acids (HETs), respectively. It is also believed that mesalamine acts aas a scavenger of oxygen-derived free radicals, which are produced in greater numbers in patients with inflammatory bowel disease.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1931
Derivatives of 5-aminosalicylic acid (mesalamine) represent a mainstay in inflammatory bowel disease therapy, yet the precise mechanism of their therapeutic action is unknown. Because tumor necrosis factor (TNF)-alpha is important in the pathogenesis of inflammatory bowel disease, we investigated the effect of mesalamine on TNF-alpha-regulated signal transduction and proliferation in intestinal epithelial cells. Young adult mouse colon cells were studied with TNF-alpha, epidermal growth factor, or ceramide in the presence or absence of mesalamine. Proliferation was studied by hemocytometry. Mitogen-activated protein (MAP) kinase activation and IkappaBalpha expression were determined by Western blot analysis. Nuclear transcription factor kappaB (NF-kappaB) nuclear translocation was determined by confocal laser immunofluorescent microscopy. The antiproliferative effects of TNF-alpha were blocked by mesalamine. TNF-alpha and ceramide activation of MAP kinase were inhibited by mesalamine, whereas epidermal growth factor activation of MAP kinase was unaffected. TNF-alpha-stimulated NF-kappaB activation and nuclear translocation and the degradation of Ikappa-Balpha were blocked by mesalamine. Mesalamine inhibits TNF-alpha-mediated effects on intestinal epithelial cell proliferation and activation of MAP kinase and NF-kappaB. Therefore, it may function as a therapeutic agent based on its ability to disrupt critical signal transduction events in the intestinal cell necessary for perpetuation of the chronic inflammatory state.
PMID:10029619 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3606885 Kaiser GC et al; Gastroenterology 116 (3): 602-9 (1999)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2021-10-24
Date Granted : 2009-06-09
Brand Name : ASACOL 800
Patent Number : 2444814
Filing Date : 2001-10-24
Strength per Unit : 800mg
Dosage Form : Enteric Coated Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2021-10-24
Date Granted : 2009-06-09
Patent Expiration Date : 2021-10-24
Date Granted : 2004-02-10
Brand Name : ASACOL 800
Patent Number : 2359812
Filing Date : 2001-10-24
Strength per Unit : 800mg
Dosage Form : Enteric Coated Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2021-10-24
Date Granted : 2004-02-10
Patent Expiration Date : 2012-06-16
Date Granted : 2002-08-20
Brand Name : PENTASA
Patent Number : 2111697
Filing Date : 1992-06-16
Strength per Unit : 1 g
Dosage Form : Suppositories
Human Or VET : Human
Route of Administration : Rectal
Patent Expiration Date : 2012-06-16
Date Granted : 2002-08-20

Patent Expiration Date : 2021-10-15
Date Granted : 2011-04-12
Brand Name : PENTASA
Patent Number : 2462905
Filing Date : 2001-10-15
Strength per Unit : 500 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2021-10-15
Date Granted : 2011-04-12

Patent Expiration Date : 2021-10-15
Date Granted : 2011-04-12
Brand Name : PENTASA
Patent Number : 2462905
Filing Date : 2004-04-05
Strength per Unit : 1g
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2021-10-15
Date Granted : 2011-04-12

Patent Expiration Date : 2020-06-08
Date Granted : 2009-05-12
Brand Name : MEZAVANT
Patent Number : 2377299
Filing Date : 2000-06-08
Strength per Unit : 1.2g
Dosage Form : Tablet (Delayed and Extended Release)
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2020-06-08
Date Granted : 2009-05-12

Patent Expiration Date : 2027-05-27
Date Granted : 2017-10-03
Brand Name : OCTASA
Patent Number : 2687130
Filing Date : 2007-05-07
Strength per Unit : 1600 mg
Dosage Form : TABLET (DELAYED-RELEASE)
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2027-05-27
Date Granted : 2017-10-03

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2027-04-13
Date Granted : 2013-11-12
Brand Name : OCTASA
Patent Number : 2648658
Filing Date : 2007-04-13
Strength per Unit : 1600 mg
Dosage Form : TABLET (DELAYED-RELEASE)
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2027-04-13
Date Granted : 2013-11-12

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2033-10-29
Date Granted : 2021-09-07
Brand Name : OCTASA
Patent Number : 2923063
Filing Date : 2013-10-29
Strength per Unit : 1600 mg
Dosage Form : TABLET (DELAYED-RELEASE)
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2033-10-29
Date Granted : 2021-09-07

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2033-04-29
Date Granted : 2023-03-21
Brand Name : OCTASA
Patent Number : 2871016
Filing Date : 2013-04-29
Strength per Unit : 1600 mg
Dosage Form : TABLET (DELAYED-RELEASE)
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2033-04-29
Date Granted : 2023-03-21

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
86
PharmaCompass offers a list of Mesalazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mesalazine manufacturer or Mesalazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mesalazine manufacturer or Mesalazine supplier.
PharmaCompass also assists you with knowing the Mesalazine API Price utilized in the formulation of products. Mesalazine API Price is not always fixed or binding as the Mesalazine Price is obtained through a variety of data sources. The Mesalazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mesalamine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mesalamine, including repackagers and relabelers. The FDA regulates Mesalamine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mesalamine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mesalamine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mesalamine supplier is an individual or a company that provides Mesalamine active pharmaceutical ingredient (API) or Mesalamine finished formulations upon request. The Mesalamine suppliers may include Mesalamine API manufacturers, exporters, distributors and traders.
click here to find a list of Mesalamine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mesalamine DMF (Drug Master File) is a document detailing the whole manufacturing process of Mesalamine active pharmaceutical ingredient (API) in detail. Different forms of Mesalamine DMFs exist exist since differing nations have different regulations, such as Mesalamine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mesalamine DMF submitted to regulatory agencies in the US is known as a USDMF. Mesalamine USDMF includes data on Mesalamine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mesalamine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mesalamine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Mesalamine Drug Master File in Japan (Mesalamine JDMF) empowers Mesalamine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Mesalamine JDMF during the approval evaluation for pharmaceutical products. At the time of Mesalamine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Mesalamine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Mesalamine Drug Master File in Korea (Mesalamine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Mesalamine. The MFDS reviews the Mesalamine KDMF as part of the drug registration process and uses the information provided in the Mesalamine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Mesalamine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Mesalamine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Mesalamine suppliers with KDMF on PharmaCompass.
A Mesalamine CEP of the European Pharmacopoeia monograph is often referred to as a Mesalamine Certificate of Suitability (COS). The purpose of a Mesalamine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Mesalamine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Mesalamine to their clients by showing that a Mesalamine CEP has been issued for it. The manufacturer submits a Mesalamine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Mesalamine CEP holder for the record. Additionally, the data presented in the Mesalamine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Mesalamine DMF.
A Mesalamine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Mesalamine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Mesalamine suppliers with CEP (COS) on PharmaCompass.
A Mesalamine written confirmation (Mesalamine WC) is an official document issued by a regulatory agency to a Mesalamine manufacturer, verifying that the manufacturing facility of a Mesalamine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Mesalamine APIs or Mesalamine finished pharmaceutical products to another nation, regulatory agencies frequently require a Mesalamine WC (written confirmation) as part of the regulatory process.
click here to find a list of Mesalamine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Mesalamine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Mesalamine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Mesalamine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Mesalamine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Mesalamine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Mesalamine suppliers with NDC on PharmaCompass.
Mesalamine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mesalamine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mesalamine GMP manufacturer or Mesalamine GMP API supplier for your needs.
A Mesalamine CoA (Certificate of Analysis) is a formal document that attests to Mesalamine's compliance with Mesalamine specifications and serves as a tool for batch-level quality control.
Mesalamine CoA mostly includes findings from lab analyses of a specific batch. For each Mesalamine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mesalamine may be tested according to a variety of international standards, such as European Pharmacopoeia (Mesalamine EP), Mesalamine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mesalamine USP).

